| MATERIALS AND METHODS |
| Photosensitizers | |
| Experiments with human serum albumin (HSA) | |
| Experiments with erythrocyte cell ghosts (ECG) | |
| Experiments with cancerous tissues | |
| Illumination conditions |
Haematoporphyrin derivative (HpD) was prepared at the Moscow Institute of Fine Chemical Technology (Russia) and dimethoxyhaematoporphyrin (DMHp) was prepared at the Moscow Institute of Biophysics (Russia). Haematoporphyrin (Hp) was purchased from Serva and Porphyrin Products, Photosan-3 (PS) was obtained from Seehof Laboratories (Germany) and Photofrin II (PF) - from QLT Cyanamid (Canada). Meso-tetraphenylporphyine tetrasulphonate (TPPS4), chlorin e6 (Cle6) and aluminum phthalocyanine tetrasulphonate (AlPcS4) were provided by Porphyrin Products (Logan, UT). All porphyrins were used without further purification. Stock solutions of photosensitizers were prepared by dissolving crude photosensitizers in 0.02 ml of 0.1 M NaOH and diluting them with phosphate buffered solutions (PBS) (pH 7.2) to concentration of 10-3 M. Stock solutions were kept in dark at 4oC. Further dilution with PBS was performed immediately before the measurements.
![]() Experiments
with human serum albumin (HSA)
Experiments
with human serum albumin (HSA)
HSA was purchased from Fluka (USA) and Reanal (Hungary). The stock solution of HSA (concentration 10-4 M) was prepared by dissolving crude HSA (Mr = 70 kDa) directly in PBS (pH 7). To prepare samples for illumination the solutions of Hp and HSA were mixed together at molar ratio of 1:1 to get 2 ml of solution.
![]() Experiments
with erythrocyte cell ghosts (ECG)
Experiments
with erythrocyte cell ghosts (ECG)
Experiments with ECG were carried out using a haematoporphyrin from Porphyrin Products, Logan, Utah. Before experiments the stock solution of Hp (10-3 M ) was diluted to 10-4 M with PBS and was used as incubation medium for erythrocyte cell ghosts (ECG). The ECG were prepared from human erythrocytes by hypotonic lysis and used at a final concentration of 0.35 mg/ml of proteins in PBS. To get the pure fraction of ECG sensitized with Hp, they were incubated with 10-4 M Hp buffered solution. After 24 h 2 ml of preparation were centrifuged (1-1.5 min, at 15400 rot/min) by a centrifuge (Janetzki TH-11). Then the pellet, separated from a supernatant, was resuspended in PBS at the same volume. This procedure was repeated four times.
![]() Experiments
with cancerous tissues
Experiments
with cancerous tissues
Hybrids of mice BDF1 (weighing 21±3 g) bearing hepatoma 22 (10±2 mm in diameter) were divided into two groups: I - control and II - experimental group. Photosensitizer photosan PS was injected into animals of group II intraperitoneally at a dosage of 10 mg/kg. After 24 h animals were killed (in the state of ether anesthesia) and the samples of hepatoma 22 were removed into physiological saline. The autofluorescence of both control (group I) and sensitized cancerous tissue (group II) was recorded ex vivo before the illumination, then the monitoring of the light-induced changes in fluorescence spectra of photosensitizer accumulated in tissues was performed.
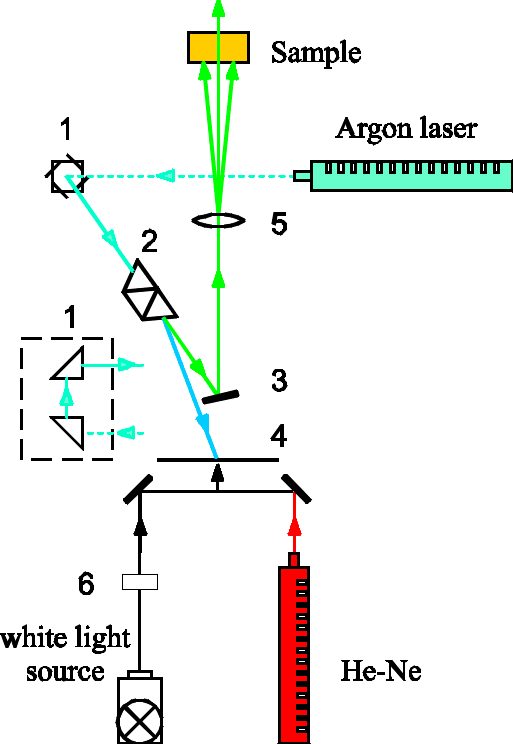
To induce phototransformation, the samples (2 ml of the solution in quartz cuvettes; path length, 1 cm) were illuminated with an Ar-ion laser (wavelength, 514 nm; light fluence rate, < 100 mW cm-2) and a He-Ne laser (wavelength, 632 nm; light fluence rate, < 25 mW cm-2) (Fig.3).
Fig.3 Setup for the irradiation of samples: 1- full reflection prisms, 2- dispersal system, 3- mirror, 4- screen, 5- condensing lens, 6- water filter.
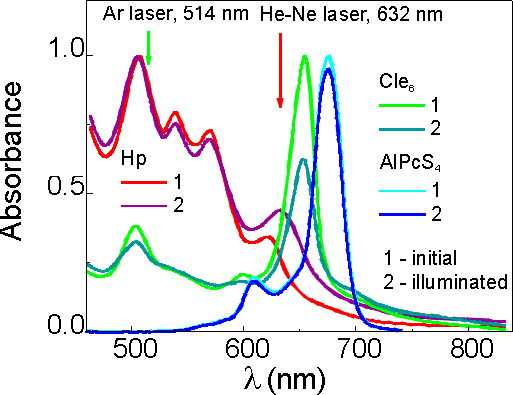
Since different photosensitizers of equal concentration have different optical densities A(l ill) at the illumination wavelength, that should be taken into account when their photobleaching efficiencies are compared. Bleaching of absorption, DA(l bl), also differs in magnitude depending on the wavelength at which it has been measured (Fig.4).
Fig.4 Normalized absorption spectra of Hp, Cle6 and AlPcS4 in PBS (pH 7.2) before and after illumination.
In order to eliminate this dependence, we used relative absorbance values for the bleaching, obtained dividing DA(l bl) by the value of the initial absorption, A(l ill), at the wavelength of illumination, and also dividing by value of initial absorption, A(l bl), at the wavelength of the measurement:
where DA(l bl) is the absorption bleaching at the wavelength of the measurement, l bl, estimated from the absorption difference spectrum after the illumination; A(l bl) and A(l ill) are the initial absorption values estimated at l bl and l ill, respectively.
The relative values of absorbance used when the rate of photoproduct formation was under evaluation were obtained by following equation:
![]()
where D A(l p) is the increase of absorption estimated at the peak of the photoproduct absorption band from the absorption difference spectrum after the illumination, A(l ill) is the initial absorbance value estimated at l ill.
![]()
|
|
|
|