| RESULTS AND DISCUSSION |
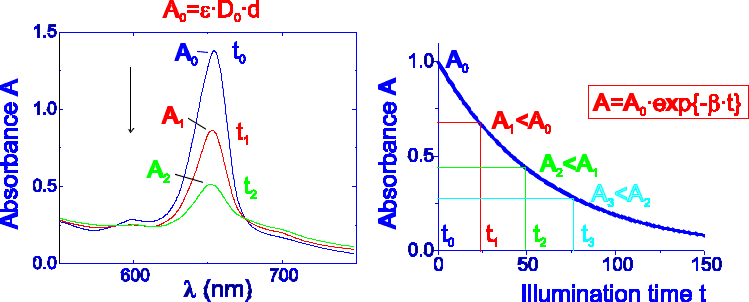
All investigated photosensitizers undergo phototransformations on illumination but photoinduced spectral changes are different. In the absorption difference spectra of AlPcS4, measured at different illumination doses, only slight photobleaching of absorption is detected (Fig.4).
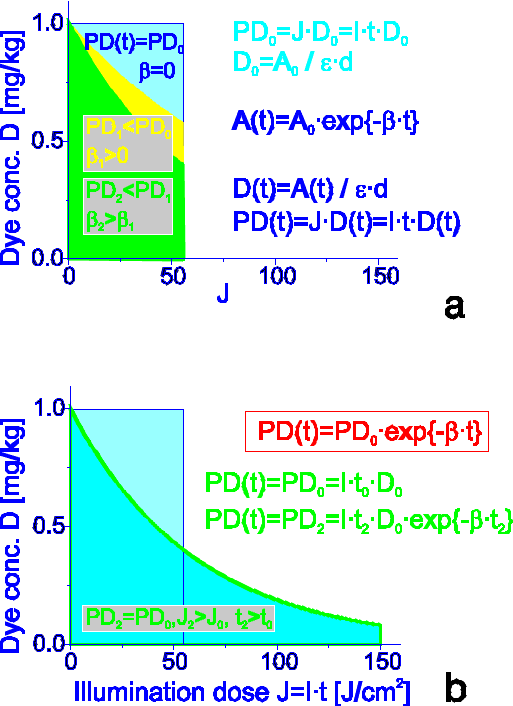
For the relatively photostable photosensitizers, AlPcS4 and TPPS4, simple geometrical definition of photochemical dose, PD=D0J0 (Fig.2), [1] (D0 is the concentration of photosensitizer, mg kg-1, J0 is the incident light dose, J cm-2) is useful, since only <1 % of the initial absorption of AlPcS4 and <10 % of the initial absorption of TPPS4 is bleached at the illumination dose of 200 J cm-2.
Changes in the absorption spectrum of Cle6 during illumination by argon ion laser presented in Fig.4 show that the dominant process is decay of the absorption. When the photobleaching of photosensitizer is significant during illumination (Fig.5), as in the case of Cle6 (>50% of the initial absorption is decreased at the illumination dose of 100 J cm-2), equation (2) is valid.
Fig.5 Significant decay of photosensitizer's absorbance occurring on illumination.
With the increase of bleaching rate constant b the lower efficiency of PTT would be expected at the same illumination dose (Fig.6) due to decrease of concentration of photosensitizer. The optimal PD thus could be reached by the appropriate increase of illumination dose (time). Fig.6 illustrates the case when the photosensitizer concentration is independent of the light dose, and when it decreases as a result of photobleaching with the rate constant b. However, the achieved PD, which is equal to the area below the curves, is the same in both cases.
Fig.6 a) photodestruction of unstable photosensitizer reflected by higher value of b finally results in lower photochemical dose; b) the same photochemical dose could be reached when illumination dose (time) is increased.
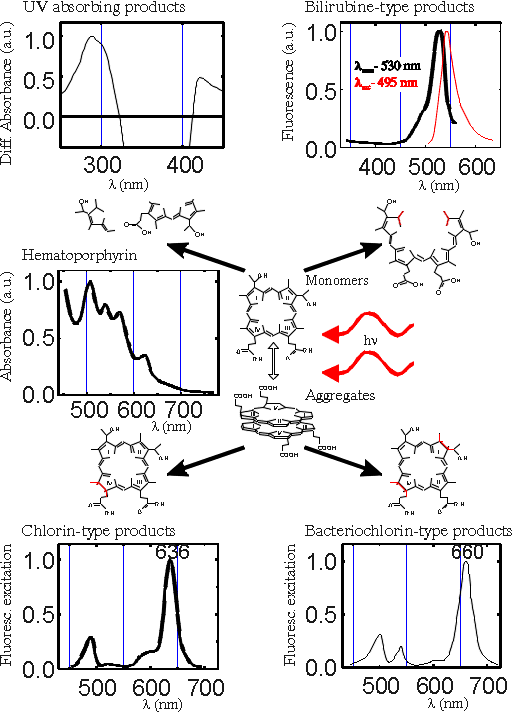
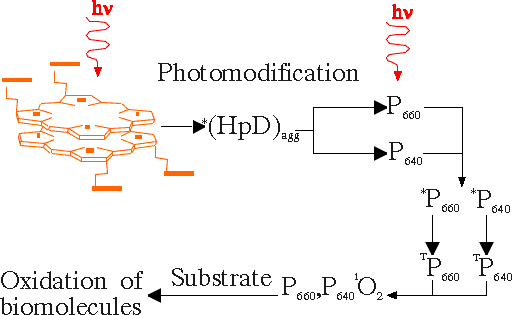
It is not always possible to approximate the photobleaching of different photosensitizers by simple exponential relations, because most of them are complex mixtures and the dependencies of their bleaching on illumination doses have different characters. As it is shown in the phototransformation scheme in Fig.7 in addition to photobleaching, another transformation takes place on illumination of the porphyrin-type photosensitizers by light doses commonly used in PTT. The latter process is slightly dependent on the oxygen concentration and is accompanied by a decrease of the fluorescence and an appearance of absorption and fluorescence bands of the photoproduct at around 640 nm [9]. The illumination of DMHp, Hp, HpD, TPPS4, PS or PF in aqueous solution causes two processes: photoinduced destruction of porphyrin macrocycle and photoinduced chemical modification leaving the porphyrin macrocycle intact. For the most part the photoinduced destruction could be explained as the opening of the porphyrin ring, leading to the increase of light absorbance in the UV and visible region (formation of mono- and di-pyrroles and biliverdin-, bilirubin-type photoproducts). Photoinduced chemical modification leaving the porphyrin macrocycle intact is related to the formation of chlorin, bacteriochlorin or chlorin-porphyrin linked systems with absorption bands in the red spectral region (Fig.7).
Fig.7 Phototransformation scheme for haematoporphyrin-type photosensitizers in aqueous medium.
Formed UV absorbing and bilirubine-type products (Fig.7) are not interesting for PTT (if they don't induce dark toxicity) since they do not absorb the red light used for clinical treatment. The chlorin-type photoproducts have a broad absorption band in the red spectral region, and therefore careful calculation of the photochemical dose requires modified formula, which includes the formation of photoproducts.
To get closer to the in vivo conditions the formation of photoproduct was also tested in model systems.
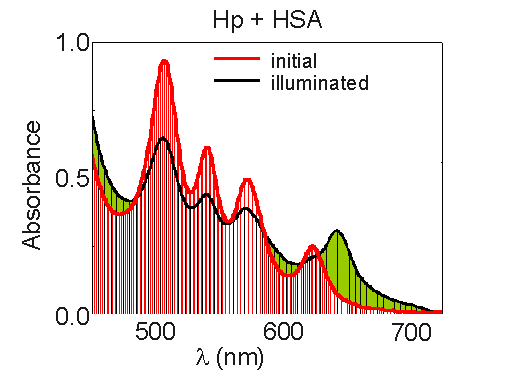
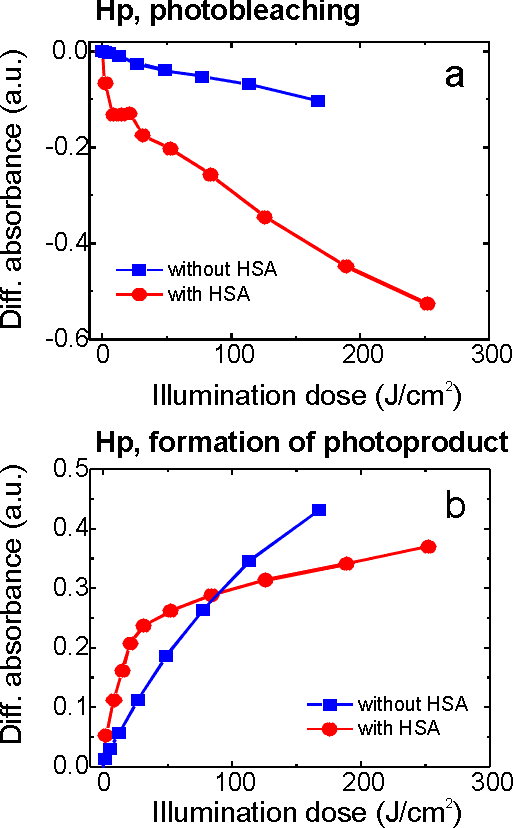
Protein-bound porphyrins play a major role in photosensitized processes occurring in vivo and affect the efficiency of photoinduced cell damage. The interaction of photosensitizer with serum albumins may affect the sensitization process, generation of active species of oxygen and monomers-aggregates equilibrium. The presence of serum proteins may also alter the photostability and mechanism of photomodification of photosensitizers. Presence of HSA increases the photobleaching rate of the photosensitizer. The observed character of the absorption bands (Fig.8) of the photobleached product remains similar to that of porphyrin - type photosensitizers in aqueous solutions, indicating that the macrocycle is still largely intact. The sizable red shift of the first Q- band infers the formation of a chlorin type photoproduct.
Fig.8 Changes of haematoporphyrin (conc. - 10-4 M) absorption spectrum in the presence of HSA (molar ratio 1:1) under illumination.
Fig. 9a depicts the absorption decay curves of free Hp and Hp complexed with HSA. As is seen‚ the photosensitizer-albumin interaction significantly accelerates photobleaching process‚ especially at low illumination doses. This tendency is more or less characteristic of all investigated photosensitizers (DMHp, PS, PF, HpD, TPPS4 and Cle6).
Fig.9 Kinetics of absorption decay registered at the maximum of third Q band (540 nm)(a) and formation of red-absorbing photoproduct registered at the maximum of formed photoproduct (640 nm) (b); the arbitrary values of absorbance for haematoporphyrin and photoproduct were obtained from absorption difference spectra by equation described above.
The photoproduct formation rate curves for Hp are shown in Fig.9b. The initial rate of photoproduct formation is higher in the presence of HSA. In general, the presence of HSA in the solution of a Hp-type photosensitizer relatively stimulates the formation of a photoproduct at low doses of illumination in comparison with a photosensitizer in the absence of HSA. After prolonged illumination the ratio between the relative absorption of a photoproduct for Hp-type photosensitizer with or without HSA changes depending on the photosensitizer and on the illumination dose.
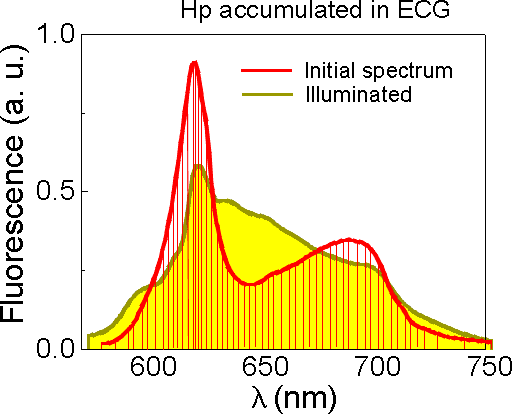
Several experiments were also carried out with Hp in the presence of erythrocyte cell ghosts (ECG), which represent a good model of real cells with a natural membrane containing lipids and proteins. The fluorescence spectroscopy technique was applied in this case. Under illumination of Hp accumulated in ECG, the disappearance of initial fluorescence bands was followed by relative increase at around 645 nm presumably caused by formed photoproduct (Fig10).
The appearance of a new fluorescence peak after illumination was also detected ex vivo in fluorescence spectra of Photosan accumulated in cancerous tissue (Fig.11).
 |
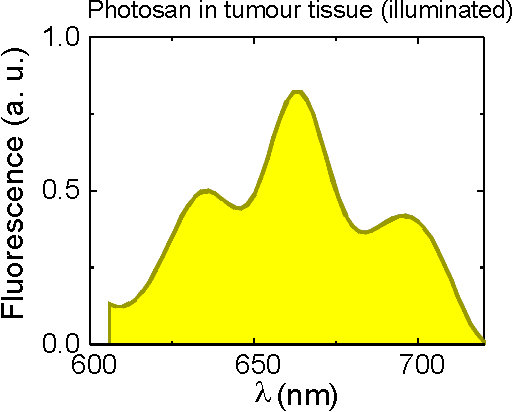 |
Fig.10 Fluorescence spectra of Hp recorded before and after illumination. |
Fig.11 A new peak observed in fluorescence spectrum of Photosan after illumination. |
It is clearly seen that the interaction of photosensitizer with molecules present in cell substrate accelerates the photoproduct formation. The photoproduct is also formed in cancerous tissue ex vivo. Therefore it might be expected that photoproduct formed during the clinical treatment could influence PTT efficiency.
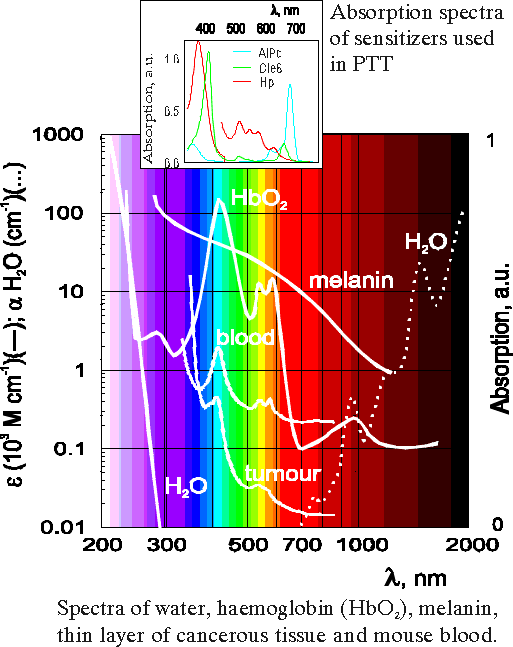
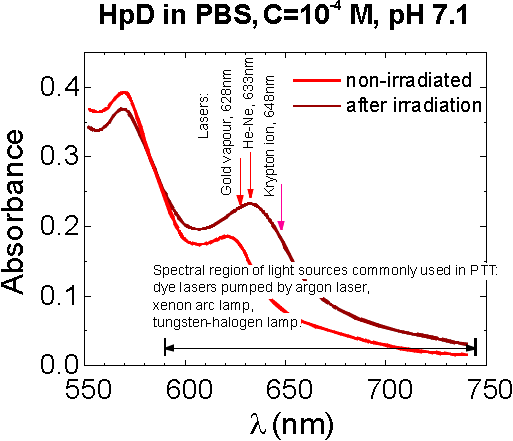
For the illumination of tumours the red light (l ~ 630 nm) is used in clinical treatment because of its deeper penetration into the tissue (Fig.12). Since the photoproduct 640 has a broad absorption band in this region (Fig.13) and shows the photosensitizing activity [17] the additional photosensitization which comes from photoproduct must be included in the calculation of the efficient photochemical doses.
Fig.12 Absorption spectra illustrating the existence of spectral zone of higher tissue transparency in the red spectral region. |
Fig.13 Light-induced (illumination by argon ion laser at 514 nm) absorption changes of HpD in the red spectral region. |
The mechanism of PTT is still unclear. It is generally accepted that the sensitization process is singlet oxygen- mediated and aggregates of porphyrin type photosensitizers are efficient in PTT because of the better accumulation in cancerous tissues. However, porphyrin aggregates are less efficient generators of singlet oxygen than monomers [18,19]. This contradiction is possible to overcome if the photomodification of photosensitizer is involved in sensitization process. Under illumination the "sandwich" type equilibrium aggregates are preferably photomodified into the chlorin-type (PhP 640) and bacteriochlorin-type (PhP 660) photoproducts [20-22]. If the first light quantum absorbed by aggregated species (bad generators of singlet oxygen) transforms them to the red-absorbing photoproducts (Fig.14), then, since the photoproduct absorbs the radiation of light sources commonly used in PTT, the next light quantum may be absorbed by the formed chlorin-type photoproduct.
Fig.14 Suggested participation of photoproducts in photosensitization process.
The chlorin-type molecules, which have higher absorption in the red spectral region and are good generators of singlet oxygen, may act as good photosensitizers. Thus, the capacity of aggregated species to generate singlet oxygen increases due to the phototransformation to chlorin-type photoproducts.
Modification of photochemical dose
The results of our experiments in model systems (photosensitizer complexed with HSA, or in a suspension of resealed erythrocyte ghosts) and in tumour tissue show that photoproducts are formed in all investigated preparations (Figs. 8,10,11). The similar light-induced changes of fluorescence spectra were also observed in vivo by other authors [13-15].
Since the formed photoproduct itself can act as photosensitizer and induce the positive photodynamic effect [17], we have made some modifications in the calculations of PD taking into account the formation of the photoproduct. So, if the decrease of photosensitizer concentration occurring with the rate constant b and being reflected by photobleaching, is caused by the photodestruction of photosensitizer with rate constant a, and simultaneous formation of red-absorbing photoproduct with rate constant k, these processes could be described with the following system of equations:
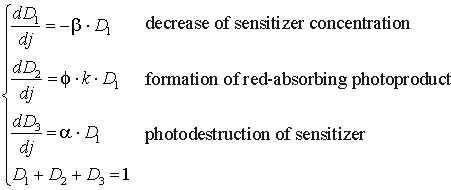
where D1, D2 and D3 are the concentrations of photosensitizer, red-absorbing photoproduct and destruction products correspondingly, b, k and a are the rate constants of the corresponding photoprocesses, f is a quantum yield of the formation of the red-absorbing photoproduct. The photochemical dose in this case will be determined by the summed effect of photosensitizer and red-absorbing photoproduct:
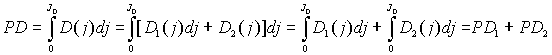
After solving the system, an integration of the obtained D1, and D2 values gives the modified formula for the calculation of PD:
where D10 is a concentration of photosensitizer before illumination.
![]()