RESULTS
Oxidation Of Oils At Elevated Temperatures (60°C)
Figure 2 shows the CL intensity (upper graph) and the UV absorbance, at both 234 nm and 270-280 nm (lower graph), of LA over time when the LA is kept at 60oC in the presence of oxygen. Figure 3 shows similar results for ML under the same conditions. An increase in the absorbance at 234 nm began almost immediately in both cases, indicating that the levels of hydroperoxides increase immediately for both LA and ML. For LA (Fig. 2, lower graph), the increase was rapid initially until a maximum was obtained after about 48 h and then the hydroperoxide concentration remained fairly constant for the remainder of the experiment, corresponding to an absorbance of 0.25-0.35. A peak in the UV spectrum at 270-280 nm appeared as a small shoulder on the main 234 nm peak and grew slowly throughout the duration of the experiment. The absorbance, and hence the hydroperoxide concentration, for ML (Fig. 3, lower graph) rose to a higher level (over 0.5 after 70 h) than that obtained for LA. It then stayed reasonably constant at this level for the remainder of the experiment. The peak at 270-280 nm did not appear until about 120 h, corresponding to the levelling off of hydroperoxide concentration (234 nm absorption), and then grew slowly.
The Chemiluminescence and Oxidation of Linoleic Acid at 60°C
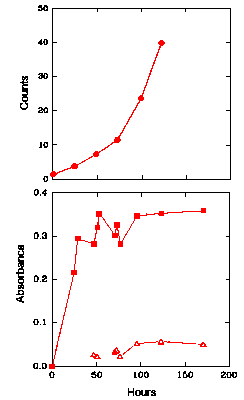
replicate
The upper graph gives the time dependence of the CL intensity observed from linoleic acid when undergoing oxidation at 60°C. The lower graph shows the UV absorbance at 234 nm (filled symbols) of linoleic acid at 60°C, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of linoleic acid at 60°C, as an indication of the formation of secondary oxidation products.
The Chemiluminescence and Oxidation of Methyl Linoleate at 60°C
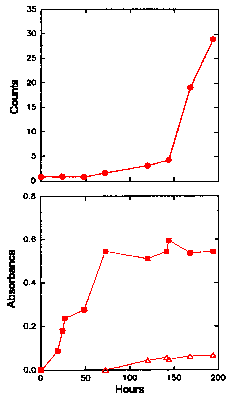
The upper graph gives the time dependence of the CL intensity observed from methyl linoleate when undergoing oxidation at 60°C. The lower graph shows the UV absorbance at 234 nm (filled symbols) of methyl linoleate at 60°C, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of methyl linoleate at 60oC, as an indication of the formation of secondary oxidation products.
In the case of LA, the CL initially increased steadily (Fig. 2, upper graph), but rose
more rapidly after about 48 h, coinciding with the levelling off of the hydroperoxide
concentration (as determined by the absorbance at 234 nm). The CL from ML (Fig. 3, upper
graph) was the same as the buffer alone for the first 50 h, then increased gradually until
about 120 h, after which it increased rapidly. The increase in CL intensity corresponded
to the levelling off of hydroperoxide concentration and the appearance of the 270-280 nm
peak. The rapid increase in CL does not appear until a much later time than for the LA.
The strong smell and colour associated with the degradation (rancidity) of LA was detected after 24 h at 60°C. After approximately 120 h the viscosity of the sample began to increase rapidly. For ML, the smell became detectable after 72 h, but the colour and increase in viscosity did not occur until after about 170 h.
Effect of Glucose on the Oxidation and Chemiluminescence of Oils
Hicks et al. (16) has shown that glucose catalyses lipid peroxidation in model systems of lipids. The present study investigated the effect of glucose on the oxidation and light emission of LA and ML micelles. Figure 4 shows the oxidation and CL emission from oxygenated solutions (50 cm3, 2 mg cm-3 ) of LA, with no catalyst added, over a period of 100 h. With no glucose added, the level of hydroperoxides (UV absorbance at 234 nm) increases linearly, while CL emission remained at the level of the buffer (35 counts s-1 ) for the entire 100 h of the experiment. No peak at 270-280 nm was observed, but the smell associated with oil deterioration developed towards the end of this experiment.
The Chemiluminescence and Oxidation of Linoleic Acid
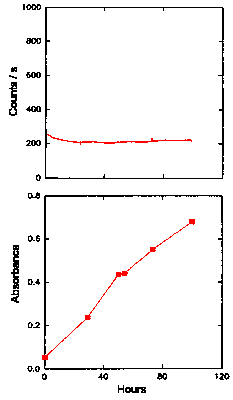
The upper graph gives the time dependence of the CL intensity observed from a solution of linoleic acid (50 cm3, 2 mg cm-3 ) when oxygenated at 25°C with no added catalyst. The lower graph shows the UV absorbance at 234 nm (filled symbols) of the solution of linoleic acid, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of the linoleic acid solution, as an indication of the formation of secondary oxidation products.
Figure 5 shows the results for LA with 20 mM glucose added. With the glucose present, the hydroperoxides accumulated rapidly, peaked at about 60 h (at an absorbance of 1.2 at 234 nm), and then decreased for the remainder of the experiment. CL was close to the base level of the buffer (approx. 35 counts s-1) for the first 25 h, before increasing to around 1300 counts s-1 after 95 - 100 h. The peak and fall in hydroperoxide concentration corresponded to the steepest increase in CL intensity. As the rate of decrease in hydroperoxide concentration eased at 80-100 h, the increase in CL also tapered off. The appearance of a peak at 270-280 nm was observed after 50 - 80 h and coincided with the reduction in hydroperoxide concentration, as expected. The appearance of secondary products, as indicated by the absorbance at 270-280 nm, thus corresponded to the reduction in hydroperoxide levels and the increase in CL. Degradation of the sample, identified by a rancid smell, was associated with the initial increase in hydroperoxide concentration, rather than their later decomposition.
The Chemiluminescence and Oxidation of Linoleic Acid Catalysed by Glucose
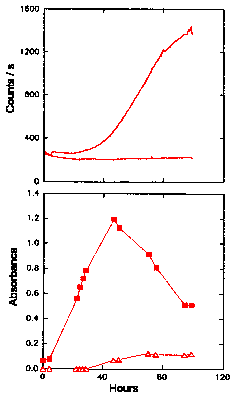
replicate
The upper graph gives the time dependence of the CL intensity observed from a solution (50 cm3, 2 mg cm-3 ) of linoleic acid when undergoing oxidation in the presence of 20 mM glucose. The CL obtained in Fig. 4, without any catalyst added, is also shown for comparison. The lower graph shows the UV absorbance at 234 nm (filled symbols) of linoleic acid (25°C) oxidized by glucose, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of linoleic acid, as an indication of the formation of secondary oxidation products.
A similar experiment involving ML was carried out using 20 mM glucose as shown in Fig. 6.
The absorbance peak at 270-280 nm was obscured during this experiment and could not be
shown in Fig. 6. In addition, the CL was low and constant for the first part of the
experiment and was thus only recorded after 130 h. The absorbance at 234 nm rose very
slowly initially (Fig. 6, lower graph), but began to increase more rapidly after 100 h,
and finally peaked at 1.2 around 220 h. The absorbance then fell slowly to about 1.0 by
385 h. The rancid smell became detectable after 140 h, once again associated with the
increase in hydroperoxide concentration. The CL began to increase linearly after about 165
h (Fig. 6, upper graph) and increased more rapidly once the level of hydroperoxides had
peaked, reaching about 800 counts s-1 after 16 days.
The Chemiluminescence and Oxidation of Methyl Linoleate Catalysed by Glucose
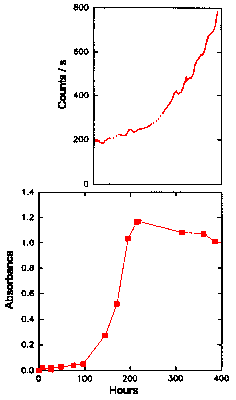
The upper graph gives the time dependence of the CL intensity observed from a solution (50 cm3, 2 mg cm-3 ) of methyl linoleate when undergoing oxidation in the presence of 20 mM glucose. The CL obtained in Fig. 4, without any catalyst added, is also shown for comparison. The lower graph shows the UV absorbance at 234 nm (filled symbols) of methyl linoleate (25oC) oxidized by glucose, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of methyl linoleate, as an indication of the formation of secondary oxidation products.
Effect of Iron (II) - NTA on the Oxidation and Chemiluminescence of Linoleic Acid
Nitrilotriacetic acid is known to be toxic to humans and has been shown to be toxic to rats when chelated to ferrous iron (23). This toxicity may be due to free radical induced peroxidation of membrane lipids, since it is known that iron (II) - NTA greatly enhances lipid peroxidation (23). The effect of iron-NTA on the oxidation and CL of linoleic acid was determined in the same manner as that for glucose, and the results are shown in Fig. 7. The absorbance at 234 nm rose sharply in the first 24 h (Fig. 7, lower graph), peaked at about 1.0 and then fell sharply to 0.60 by 48 hours. The CL (Fig. 7, upper graph) increased rapidly after about 12 h, rising to a level of 2200 counts s-1 after 60 hours. The detection of a rancid smell once again followed the formation of hydroperoxides. These results are similar to those using glucose as a catalyst, where the CL is closely related to the decrease in the levels of hydroperoxides in the system. The maximum hydroperoxide concentration was slightly less than the levels observed with glucose as catalyst, but the CL intensity was greater.
The Chemiluminescence and Oxidation of Linoleic Acid Catalysed by Iron (II) - NTA
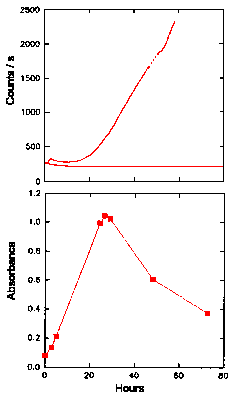
The upper graph gives the time dependence of the CL intensity observed from a solution (50 cm3, 2 mg cm-3 ) of linoleic acid when undergoing oxidation in the presence of 100 mM iron (II) nitrilotriacetate. The CL obtained in Fig. 4, without any catalyst added, is also shown for comparison. The lower graph shows the UV absorbance at 234 nm (filled symbols) of linoleic acid (25oC) oxidized by iron (II) nitrilotriacetate, as an indication of the formation of hydroperoxides. Also shown in the lower graph is the UV absorbance at 270-280 nm (unfilled symbols) of linoleic acid, as an indication of the formation of secondary oxidation products.
 Discussion |
|||
 Abstract |
Introduction |
 Materials and methods |
 References |
![]()