4. RADIATION CHEMISTRY OF WATER
The radiation chemistry of water has been studied since the discovery natural radioactivity. Early workers starting with Bequerel observed that radium emanations decompose water into the hydrogen and oxygen. This reaction was explained by dissociation of water molecules into the constituent atoms. Further studies with deaerated water led to the identification of the molecular yield consisting of H2 and H2O2 which was attributed to the recombination reactions of primary H· and OH· radicals. The slow decomposition of H2O2 explains the formation of O2. The irradiation of inorganic and organic compounds in aqueous solution usually generate more products and higher yields under oxygenated conditions compared to oxygen-free solutions. The enhancing effect of oxygen was attributed by the formation of hydroperoxy radicals HO2· from reactions of H· atoms with molecular oxygen. HO2· is the acidic form of superoxide O2- with pKa = 4.88. This straightforward theory was unchallenged until the early 1960s. Very puzzling questions arose when accurate rate constant measurements with strong gamma-ray sources were inconsistent with the assumption that H· atoms are the dominant reducing species in the radiolysis of deaerated water, e.g., the dependence of the rate constants on ionic strength are indicative of a negatively charged "H atom". The new species was first referred to as H', an entirely unsatisfactory situation. Pulse radiolysis measurements of Hart and Boag in 1962 led to the identification of the hydrated electron (e-aq). Irradiation of pure water with short pulses of fast electrons from a LINAC generated a very strong, broad optical absorption band peaking at 720 nm attributed to e-aq (Fig. 16). Although the role of hydrated electron in radiation chemistry and photochemistry is now fully accepted, it was a dubious concept before 1962. The earlier assumption was that photochemical reactions in solution are initiated by excited molecular states and free radicals originating from rupture of chemical bonds. In the 1950’s Platzmann postulated that free electrons can be stabilized in water based on theoretical considerations. Studies on continuous photolysis of aqueous halide ions and phenolate anion by ultraviolet light performed by Stein and coworkers in 1962 were explained by postulating that that hydrated electrons are generated in the initial act. This conclusion was confirmed by Grossweiner and colleagues in 1963-65 in which
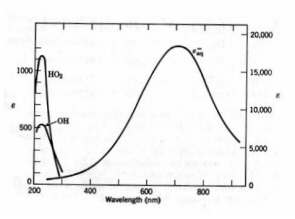
Figure 16. Absorption spectra of water radiolysis intermediates measured by pulse radiolysis. The extinction coefficient of e-aq is given on the right and that of OH and HO2 radicals on the left. (Adapted from Spinks and Woods.)
the same e-aq absorption generated by electron pulse radiolysis was identified n flash photolysis spectra of aqueous iodide ion and many aromatic compounds including amino acids. (Flash photolysis was the precusor of pulse radiolysis in which photochemical reactions are initiated by an intense light flash and identified by spectroscopic techniques.) The decay of the e-aq absorption is accelerated by agents which are expected to react with electrons including H+ , O2 , and N2O. The fast reaction of e-aq with N2O is conclusive because H
· atoms do not react with N2O:N2O + e-aq + H+ v N2 + OH·
According to the current model of water radiolysis, the primary act involves the ionisation of water leading short-lived H2O+
radical-cations, fast electrons, and electronically excited water molecules (H2O*):H2O Y e-, H2O+, H2O*
The fast secondary electrons lose energy by a sequence of interactions with the medium until they attain thermal energies after about 10-11 s. The thermalized electrons are then solvated by dielectric interactions with neighboring water molecules to form e-aq . A suggested physical picture of e-aq is a free electron in a solvent cavity surrounded by a sheath of oriented water dipoles. From the chemical viewpoint, e-aq is the is the anion of the H
· atom:e-aq + H+ W H·
with pKa = 9.7. e-aq is the strongest known reducing species with a reduction potential Eo' = - 2.9 V at pH 7. e-aq is converted to O2- in oxygenated systems, which is a strong oxidizing agent and the precursor of hydrogen peroxide. The fate of the other primary radiolysis products has been deduced from indirect evidence. H2O+ ions are unstable and decompose within 10-13 s to form OH
· radicals by transferring a proton to an adjacent H2O molecule:H2O+
+ H2O v OH· + H3O+A low yield of H
· atoms is attributed to the dissociation of electronically excited water molecules:H2O* v H· + OH·
Additionally, H2O
* may dissociate to give e-aq and OH· . The molecular yield is attributed to fast radical-radical reactions in the spur:H
· + H· v H2H
· + OH· v H2OOH· + OH· v H2O2
OH· + H2O2 v HO2· + H2O
The relative yields of the water radiolysis products depend on the pH and LET of the radiation. Typical G-values are given in
Table 5. The reducing radicals (e-aq and H· ) and the oxidizing radical (OH· ) dominate for low LET radiation with a low molecular yield of H2 and H2O2. The higher molecular yield for high LET radiation results from spur reactions. Hydrated electrons are unstable in strongly acidic solutions and the molecular yield is even higher. Many homogeneous bimolecular reactions between the primary radiolysis radicals have been identified and measured by pulse radiolysis. They include the reactions indicated above as well as the e-aq reactions:e-aq + OH· v OH-
e-aq + H· v H-
e-aq + e-aq v H2 + 2OH-
| Radiation | G(-H2O) | G(H2+H2O2) | G(e-aq) | G(H) | G(OH) |
| x-rays and fast electrons 0.1-20 MeV |
4.08 pH 3-13 |
1.13 | 2.63 | 0.55 | 2.72 |
| 12 MeV alpha | 2.84 pH 7 |
2.19 | 0.42 | 0.27 | 0.54 |
| Polonium alpha, 3 MeV | 3.62 pH 0.46 |
3.02 | 0 | 0.60 | 0.50 |
Table 5 Radical and molecular product yields in irradiated water. (Adapted from J. W. T Spinks and R. J. Woods.)
The rate constants of these radical-radical reactions are close to diffusion-limited, ranging from 5 x 109 to 3 x 1010 liter/mol-sec. The water radiolysis radicals react with a very large number of inorganic and organic solutes. Some examples of fast reactions are as follows: e-aq reacts very rapidly with reducible metal ions (Cd2+, Cu+, Fe2+), unsaturated aliphatics (acetylene, acetaldehyde, oxalic acid, tetranitromethane), halogenated hydrocarbons (chloroform), aromatics (benzophenone, nitrobenzene), sulphur compounds (cysteine, cystamine), and nucleic acid bases (purine, thymine, uracil); OH- reacts rapidly with oxidizable ions (Br-, CNS-, I-. Fe(CN)64-) and organic compounds of various structures (alcohols, acrylamide, formate, ethyl acetate, nitromethane, pyridine, benzene, aniline, phenol), sulphur compounds (cystine, cysteamine,), aromatic amino acids (phenylalanine, tyrosine, tryptophan), and diverse other biological molecules (ribose, thymine, uracil, glucose). The reactions of H
· and HO2· are similar to OH- but usually slower. The radiolytic oxidation of Fe2+ to Fe3+ has been employed since the 1930s for measurements of absorbed dose in radiation chemistry. The Fricke dosimeter consists of air-saturated 1 mM Fe2+ at pH 0.46. The conversion of Fe2+ to Fe3+ is measured by spectrophotometry. The accepted yield is G(Fe3+) = 15.5 for low LET radiations. Some other aqueous systems utilized as radiation dosimeters include bromide ion, ceric sulfate, ferrocyanide, formic acid, ethanol, and ultrapure water.4.1. Spur Reactions
Spur reactions are important at high solute concentrations as exist in a biological matrix. Low LET radiation such as gamma-rays and fast electrons generate small isolated spurs (
Fig. 8). Heavy charged particles generate dense tracks of closely spaced spurs (Fig. 6). The basic features of spur reactions are deduced by solving the classical time-dependent diffusion equation for an isolated spur assuming competition between three reactions: the bimolecular reactions of the primary radiolysis radicals, pseudo-first order reactions of the primary radicals with high concentrations of scavengers in the spur, and diffusion of the primary radicals into the external medium where they may recombine and react with various agents. In the method of "prescribed diffusion" it is assumed that low LET radiation generates an isolated spherical spur with a gaussian (bell-shaped) distribution of radicals in the radial direction. This geometry is referred to as a "point source" in diffusion theory. A certain fraction of the initial species react with each other within the spur leading to the molecular yield. The remaining radicals escape to the external medium where homogeneous reactions take place. High LET radiation is modeled by a long cylindrical spur. This geometry corresponds to a "cylindrical source". The analysis predicts a high molecular yield without escape of radicals to the external medium. Thus, the high molecular yield for high LET radiation is consistent with the inability of the primary radicals to escape from dense cylindrical spurs while the lower molecular yield for low LET radiation is consistent with formation of isolated spherical spurs in which the primary radicals can escape to the external medium. The actual situation is complicated by the overlap of spurs and the different rate constants for the primary reactions. Computer simulations are employed in current work for more accurate calculations.5. RADIATION EFECTS IN VARIOUS SYSTEMS
This section reviews the effects of ionising radiation on systems of practical importance. The examples illustrate several important reactions and specialized experimental techniques
5.1. Gases
Radiolysis of a gas may generate ions, excited atoms and molecules, and free radicals. Many experimental techniques have been employed to study gas-phase reaction mechanisms, including permanent product analysis, measurements of ion currents, mass spectrometry, pulse radiolysis, the effects of scavengers, and isotopic labeling. The frequent types of reactions include direct formation of electronically excited molecules:
M Y M*
and indirect excitation by neutralization of an ion-pair:
M Y M+ + e- v M*
Excitation energy may be transferred to another molecule:
M* + m v M + m*
which is called a "collision of the second kind". Alternatively, excitation energy may be emitted as optical fluorescence:
M* v M + light
or lost by collisions with other molecules or the walls of the containing vessel. Excited molecules may decompose into radicals:
M*
v A· + B·or molecular products:
M* v C + D
Radical and molecular products may be generated in an excited state. They are referred to as "hot" and can initiate otherwise endothermic (energy deficient) reactions. There is a voluminous literature on gas-phase radiation chemistry and considerable controversy as to the details. Several examples are summarized to illustrate the reaction mechanisms that have been deduced.
The radiolysis of molecular oxygen has been of continuing interest for its own sake and the importance of this reaction in the atmosphere. Five primary reactions have been identified:
O2 Y O2+ + e-
O2 Y O+ + O· + e-
O2 Y 2 O·
O2 Y O2*
Electron-ion recombination generates oxygen atoms:
O2+ + e- v 2O·
O+ + e- v O·
Electron capture generates O2- ions:
O2 + e- v O2-
Ozone is formed by the reaction:
O· + O2 + M v O3 + M
where M is another molecule of oxygen or the wall of the reaction vessel which acts as a "third body" to remove the excess energy and conserve momentum. Thus, the overall radiolysis reaction converts molecular oxygen to ozone.
Nitrous oxide is used as a dosimeter in the gas phase with G(N2) = 10.2 from 200-600 torr. The initial steps are ionization and excitation:
N2O Y N2O+ + e-
N2O Y N2O*
followed by
:N2O+ v NO+ + N·
N2O+ + e- v N2O*
N2O + e- v N2 + O-
N2O* v N· + NO·
N2O* v N2 + O·
Many tertiary reactions of the radical products are feasible. The overall radiolysis reaction is:
12 N2O
Y 10.2 N2 + 4.2 O2 + 3.6 NOMany other gas phase reactions have been investigated including conversion of para-hydrogen to ortho-hydrogen, tritium labeling, radiolysis of molecular hydrogen, water vapor, mixtures of oxygen and nitrogen, carbon monoxide, synthesis and decomposition of ammonia and hydrogen bromide. Some organic compounds which have been studied include methane, ethane, ethylene, acetylene, propane and other saturated hydrocarbons, cyclohexane, and benzene.
5.2. Biological Compounds
Practically every type of physical and chemical assay technique has been utilized for radiation studies on biological molecules, including, ultracentrifugation, chromatography, electrophoresis, x-ray diffraction, chemical product analysis, and functional changes in active biomolecules. A limitation of these methods is that the intermediate stable products are continuously radiolysed. For example, gamma-ray irradiation of aqueous glycine, the simplest amino acid, leads to many products, including high yields of NH3, H2, CO2, CHOCO2H, HCHO, and CH2NH2. Two specialized techniques probe the early radiolysis stages . Pulse radiolysis measurements at room temperature provide information about the primary and secondary reaction species involved in indirect action. Information about the early stages of direct action has been obtained from electron paramagnetic resonance (EPR) of irradiated single crystals, powders, glasses and frozen solutions.
Pulse radiolysis studies lead to optical absorption spectra and kinetics information which assist in the identification of transient radiolysis products. The reactions of OH
· radicals are promoted under N2O saturation which converts eaq- to OH·; eaq- reactions are favored by adding t-butanol, which converts OH· to relatively non-reactive alcohol radicals. OH· radicals react with aliphatic alcohols and carbohydrates by abstraction of hydrogen atoms from positions which are a- to a hydroxyl group. One such reaction for glucose is: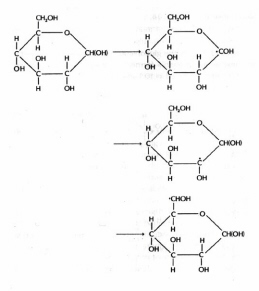
Figure 17. Radiolysis of glucose. Primary OH radicals abstract hydrogen atoms from positions alpha to hydroxyl groups.
Similar reactions occur at the other CH(OH) ring sites and the CH2OH side chain. A multiplicity of reactions occur in irradiated polysaccharides. Practical consequences of radiation exposure are that paper becomes brittle, cellulose fibres and plastics lose strength, and fruits and vegetables become soft owing to degradation of the pectin component. Proteins contain many reactive sites for the primary water radiolysis intermediates. eaq- reacts with histidine, cystine, asparagine, arginine, and the aromatic amino acids, tryptophan, tyrosine, and phenylalanine. OH
· reacts with all of the common amino acids at the peptides bonds and also at functional groups, e.g., the addition of OH· to the indole ring of tryptrophan. The -SH and –S-S- groups of sulphur-containing amino acids are highly reactive. H· reactions are much slower than OH· for the aliphatic amino acids, except the aromatic and sulphur-containing amino acids. OH· radicals add to the ring of nucleic acid bases forming radicals that react rapidly with molecular oxygen; eaq- reacts very rapidly with nucleic acid bases and is non-reactive towards the sugar and phosphate moieties. In general H· reactions with nucleotides are slower than OH· . Estimations for DNA based on results with the constituents indicate that 25% of OH· radicals react with deoxyribose units and 75% react approximately equally with the nucleic acid bases. Irradiation of DNA also leads to rupture of sugar-phosphate bonds between two nucleoside residues or single strand breaks. A significant fraction of the apparent strand breaks are induced by the assay technique of alkaline sucrose gradient centrifugation. These "alkali-labile" bonds are not scored as strand breaks in vivo.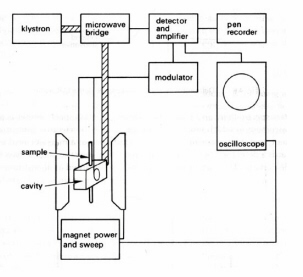
Figure 18. Diagram of an EPR apparatus. Unpaired electrons in the sample are aligned by a strong external magnetic field which is oscillated over a small range of field strengths and displayed on the x-axis of the oscilloscope. Fixed frequency microwave radiation delivered to the sample cavity by the waveguide induces magnetic transitions between "spin-up" and "spin-down" states of the electrons. The power exchanged between the electrons and the microwaves is amplified and displayed on the y-axis of the oscilloscope. (Adapted from Swallow.)
The early stage radiolysis reactions in dry biological powders and crystals have been identified by electron paramagnetic resonance (EPR). Electrons possess an intrinsic angular momentum referred to as spin. A magnetic moment is always associated with a classical rotating charged body. (Magnetic fields originate from magnetic dipoles, e.g., a bar magnet and a coil of wire carrying current. The magnetic moment is proportional to the strength of the source.) Similarly, the intrinsic angular momentum of an atomic or nuclear particle has an associated magnetism, although the classical concept of "rotation" is only pictorial. The ratio of the magnetic moment to the angular momentum is the gyromagnetic ratio. The gyromagnetic ratio of the electron is about 2000 times higher than that of protons, neutrons, and magnetic nuclei. Not all nuclei are magnetic because the magnetic moments of the constituent protons and neutrons can cancel out. Magnetic nuclei in biological materials include 13C, 14N, 19F, and 31P. EPR is based on the "anomalous" Zeeman effect in which an external magnetic field interacts with intrinsic magnetic moments of unpaired electrons leading to wavelength shifts in atomic and molecular optical spectra. (The "normal" Zeeman effect refers to spectral shifts originating from orbital electron motions.) EPR is a non-optical technique in which energy transfer between unpaired electrons and an external magnetic field is measured with a sensitive detection system. The magnetic moment of an electron is quantized in two orientations, "spin-up" and "spin-down". The magnetic energy transferred between the electrons and the magnetic field corresponds to the energy difference between the two spin states:
(28)
: DE = 2meBwhere
me is the magnitude of the magnetic moment of the electron and B is the strength of the effective magnetic field. In EPR, a small sample is located in a microwave cavity which lies between the poles of a strong electromagnet (Fig. 18). A transverse microwave field generated by a klystron is applied to the cavity which induces the spin transitions. The maximum power absorption takes place at exact resonance:(29)
: hf = 2meBwhere f is the microwave frequency. In practical units, the resonance magnetic field of a free electron is 1.25 tesla (12,500 gauss) at the standard Q-band microwave frequency of 35 Ghz. For purely technical reasons, the 1st derivative of the absorption spectra is recorded in EPR measurements. A single absorption band (a "hill") appears as a "hill and valley" pair. In EPR terminology, this spectrum is referred to as a "singlet". The EPR spectrum of a free electron consists of a singlet corresponding to transitions between spin-up and spin-down. However, the perturbing effects of nearby magnetic nuclei may induce a substructure to EPR spectra (the hyperfine interaction ) because the magnetic fields of the nuclei add or subtract from the external magnetic field depending on the orientation of the nuclei in the external magnetic field. In general, a nuclear spin of magnitude I has (2I + 1) orientations in a magnetic field. Each orientation is characterized by a quantum number mI. The
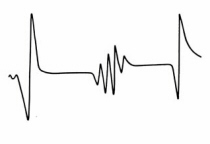
Figure 19. EPR spectrum of solid methane showing the H atom doublet and methyl radical quartet, (Adapted from O’Donnell and Sangster).
allowed values of mI are: -I, (-I+1), (-I+2), …..(I-2), (I-1), I. As a specific example, the proton in a hydrogen atom has a nuclear spin I = ½ and mI = + ½ and – ½. The EPR spectrum of an unpaired electron on an H atom consists of two equal intensity lines while that of an unpaired electron on an N atom has three lines in the intensity ratio 1:2:1. The nuclear spin arrangements are: (
) (¯ ) ( ¯) (¯ ¯). In general N equivalent protons lead to (N +1) EPR lines. Carbon nuclei are non-magnetic (I = 0) and the EPR spectra of unpaired electrons on a 12C atom originates from the effects of the adjacent protons. The EPR spectrum of solid methane irradiated with gamma-rays at 4° K in Fig. 19 shows the H atom doublet (one proton) and a quartet (3 protons) due to methyl radicals. EPR measurements on gamma-irradiated proteins containing sulphur generate complex spectra at low temperature which change to a two-component spectrum at room spectra. One component is a doublet attributed to an a-hydrogen on a polypeptide chain:¾ NH-C·H-CO¾
The second component is a small broad peak extending to low magnetic fields attributed to sulphur radicals:
¾
C-(H2)S·Since I = 0 for 32S, the hyperfine interaction may result from adjacent protons and the N atoms of peptide bonds. Detailed studies have shown that these radical species are secondary irradiation products.
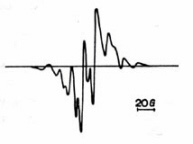
Figure 20. EPR spectrum of thymine irradiated with gamma-rays at room temperature. (Adapted from Alexander and Lett.)
Irradiated DNA leads to base radicals formed by hydrogen addition at the 5,6-double bond of pyrimidines and at positions 2 or 8 of purines. The most prominent feature is an eight-line spectrum from thymine (
Fig. 20). The secondary reaction leading to this radical is: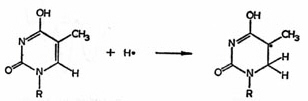
Figure 21. Thymine radicals in irradiated DNA are formed by reactions with primary H-atoms.
In the related technique of nuclear magnetic resonance (NMR) the magnetic moments of protons or other magnetic nuclei are aligned by an external magnetic field and exchange energy with EMR in the radiofrequency range. The proton resonance frequency is 42.6 MHz at 1.0 tesla. NMR studies on biomolecules are used for definitive determinations of nuclear structure and as a probe for rate processes. Magnetic resonance imaging (MRI) is based on the effect of the local environment on the relaxation rate of tissue protons aligned by a strong magnetic field.
Radiation Modifiers: The effect of ionising radiation on biological organisms is changed by radiation protective and sensitizing agents. Easily oxidized compounds act as radiation protective agents especially sulphydryls. These agents are believed to reverse oxidative damage to DNA by transfer of electrons or H-atoms to the damage sites. Incorporation of 5-bromouracil in DNA enhances radiation damage by easily split C-Br bonds. Exogenous N-ethylmaleimide acts as a radiation sensitizer by binding sulphydryl. Cells possess enzymic systems that repair sub-lethal radiation damage. Certain types of primary damage are "fixed" by molecular oxygen while other types are not affected by oxygen. The oxygen enhancement ratio (OER) is defined as the of the radiation sensitivity of a biological system in the absence of oxygen relative to that with oxygen. A typical OER is about 3 for mammalian cells exposed to gamma-rays. High LET radiation has a lower OER and higher RBE than low LET radiation. The reason for this is that the "heavier" primary damage from high LET radiation is less repairable and therefore less likely to be affected by oxygen. This result has led to the use of high LET radiation in cancer therapy. The cancer cells in the core of a solid tumor have a lower oxygen concentration than well-oxygenated normal tissues which limits the radiation dose. This "trade-off" is quantified by the therapeutic ratio (TR) defined as the ratio of the normal tissue tolerance dose to the tumor lethal dose. The potential advantage of high LET radiation for cancer therapy is that the damage to cancer cells is comparable to well-oxygenated normal tissues which increases the TR. Exogenous chemical agents referred to as anoxic sensitizers such as the drug metronidizole are intended to that mimic the effects of oxygen and sensitize the more poorly oxygenated tumor regions. The technique is not widely used, however.
5.3. Materials
Radiation effects in inorganic crystals and glasses provides information about the damage mechanisms in more complex materials. Lattice imperfections present in even the purest single crystals are an essential factor in the radiation damage
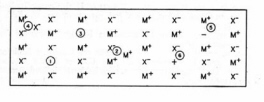
Figure 22. Depiction of colour centres in an alkali halide crystal MX. (1) cation vacancy, (2) cation interstitial, (3) anion vacancy, (4) anion interstitial, (5) F centre, (6) V1 centre. (Adapted from O’Donnell and Sangster.
)mechanisms. It has been known since the 1930s that x-rays induce visible colouration in alkali halide crystals, e.g., LiCl turns bright yellow and KCl turns sky blue. The responsible entities are termed colour centres. The radiation-induced colour can be bleached out by heating the crystal or by exposure to visible light. From measurements of optical spectra, EPR, and electrical conductivity in very pure crystals, it was shown that these colour centers consist of electrons trapped in anion vacancies normally present in the crystal. They are referred to as F-centres. An F-centre resembles an H atom because an anion vacancy in a crystal has a net positive charge. The ionization energy is shifted from 13.6 eV in the free H atom to 2.25 eV in KCl owing to the shielding effect of the dielectric medium. F-centres are formed also by heating an alkali halide crystal in an alkali metal vapor. This process generates new anion vacancies which then trap the additional electrons provided by the alkali metal. Illumination of crystals containing F-centres with visible light generates new entities absorbing in the near-IR region. They are referred to as F' -centres and are identified with two electrons trapped in an anion vacancy. The F' -centre resembles an H- ion in a dielectric medium. Hard radiations produce copious yields of F-centres by creating new anion vacancies. A V1-centre is a "hole" (positive charge) trapped in a cation vacancy. Other types of colour centres have been found in alkali halide crystals and identified with various types of lattice imperfections and impurities (
Fig. 22).Colouration effects in irradiated glasses are believed to involve colour centres, although the identification of the absorption bands with specific entities is less satisfactory. In addition to optical effects, ionising radiation induces chemical changes in most organic solids, e.g., gamma-radiolysis of solid potassium chlorate generates oxygen, ClO2-, Cl-, ClO4-, plus small yields of H2 and other products. The darkening of a silver halide photographic emulsion by ionising radiation is used in personal protection monitors. Radiation-induced changes in organic polymeric materials are of industrial importance. The principal effects are scission of main-chain bonds (degradation) and chemical bonding (cross-linking). Graft co-polymerisation is used to polymerize a second monomer on an existing polymer. This technique is especially useful for changing the surface properties of a polymer without altering the base material, e.g., to attach biological molecules to a polymer base. Production of crease-resistant clothing by graft co-polymerization on cotton or wool is an important application. Irradiation of solids with neutrons and heavy particles induce atomic displacements in solids which affect many physical properties. The rapid release of energy when a neutron is absorbed also generates a heat pulse which can affect the local structure of a metal. Irradiated solids undergo changes in dimensions, electrical conductivity, thermal conductivity, and mechanical properties. In general, metals are relatively resistant to irradiation by light particles and photons, as are ceramics, inorganic lubricants, and concrete. The radiation stability of a plastic depends on the composition, e.g., epoxy resins are very stable while irradiation of neoprene liberates corrosive HCl. Radiation sterilization of food has been of increasing interest owing to recent problems with bacterial contamination. The shelf life of fish, shell fish, poultry, and meat can be increased to about 30 days after exposure to 3-10 kGy. Higher dose levels can lead to "off" flavour and nutritional changes especially in fatty foods. These effects are reduced by vacuum-packaging and sub-freezing irradiation. Typical radiation sources are 60Co
g-rays (1.25 MeV) and 137Cs g-rays (0.66 Mev) and b-rays (0.24 Mev average). The induction of artificial radioactivity in an irradiated food product is negligible at energies less than 10 MeV.5. COMMENTS ON THE CURRENT STATUS OF RADIATION RESEARCH
Ionising radiation exemplifies one of the conundrums of modern science – the same entity can be lethal and life-preserving. This dilemma extends to the practical applications such as the pros and cons of nuclear power and radiation sterilization of food products. Radiation research started in 1896, four years prior to the Planck back body radiation theory and 17 years before the Bohr atomic model! The first report of human injury from x-rays occurred in the same year. The earliest researchers discovered that ionising radiation decomposes water, darkens glass, affects plants, and can be used for medical imaging and cancer treatment. Later advances had to wait for future developments in other fields, e.g., the key genetic role of DNA. Radiation research changed from a relatively arcane field to one of major importance after World War II. National laboratories were established with large research programs on radiation chemistry, radiation damage to materials, and radiation biology. The period from 1950-1970 was the "golden age" of radiation research. New radiation sources were available for research applications including the nuclear reactor, 60Co irradiator, and microwave LINAC. Important discoveries made in this period include the mechanism of water radiolysis, identification of the hydrated electron and many other reactive free radicals, the structure of colour centres in solids, techniques of radiation polymerization, and the role of DNA in radiation biology. Scientists from universities, government laboratories, and industry interacted regularly at meetings of the Radiation Research Society, Gordon Research Conferences, and the quadrennial International Congress on Radiation Research. Recent progress has been slower. The national laboratories, where much of the pioneering radiation research was performed, have moved from basic to more applied areas. There has been a shift of research interest away from ionising radiation to molecular photophysics and photochemistry stimulated by the development of the laser. Much of the current interest in medical radiation physics is directed towards practical issues in radiotherapy of cancer. The rapid growth of the nuclear power industry has reversed. At present ionising radiation research suffers from an identity problem. Dissemination of information in this field is widely diversified. Although a few journals are still dedicated to radiation research, much of the recent work is published in more general biology, chemistry, and physics journals. Fortunately, a useful monthly compilation of the current literature is distributed online by the
Radiation Laboratory at the University of Notre Dame. A resurgence of interest in basic radiation science may require the development of new techniques. The x-ray laser may make it possible to investigate ionising radiation interactions with the high selectivity now available in the optical spectrum. Computer simulation of very early events has not been exploited to the same extent as for thermal and optical phenomena. The most important factor, however, would be a recognition that unsolved problems in radiation science are important and interesting.Glossary
Activity: the number of nuclear decays by a radioactive element per unit time.
Alpha-rays or alpha-particles (
a): energetic helium nuclei emitted by a radioactive nucleus.Anoxic sensitizer: a chemical agent that mimics the sensitizing effect of molecular oxygen in radiation therapy of poorly oxygenated tumor tissues.
Atomic number (Z): the number of protons in an atomic nucleus.
Auger effect: - absorption of a photon by an atom in which two electrons are ejected, one from the inner L-shell and the other from the outer K-shell.
Beta-rays or beta-particles (
b): fast electrons emitted by a radioactive nucleus.Betatron: a resonance device in which electrons are confined to a fixed circular orbit by a transverse magnetic field and accelerated by electric induction induced by oscillating the magnetic field.
Bragg peak: a radiation damage maximum near the end of a heavy charged particle track.
Bremsstrahlung: broad-spectrum x-rays generated by the rapid deceleration of fast electrons striking a metallic target.
Cage effect: fast dissipation of excess kinetic energy of free radicals generated by ionising radiation induced by interactions with adjacent solvent molecules.
Colour centres: microscopic entities responsible for the colouration of crystals and glasses by exposure to ionising radiation.
Competition method: a steady-irradiation technique for measuring free radical reaction rate constants by comparison to a standard solute.
Compton effect: interaction of a photon with an atom in which kinetic energy is transferred to the atom and a longer wavelength photon is scattered.
Cross section: a hypothetical area which is proportional to the probability of a specific microscopic event.
Curie (Ci): unit of radioactivity defined as 3.7 x 1010 decays per second.
Cyclotron: a resonance device in which heavy charged particles are confined to circular orbits by a transverse magnetic field and accelerated by a fixed-frequency alternating high-voltage electrostatic field.
D37: the radiation dose at which approximately 37% (36.79%) of the initial entities are undamaged.
Deuteron: nucleus of the hydrogen isotope 2H1.
Diffusion-limited reaction: a reaction in which the rate is controlled by the diffusional properties of the reactants in a fluid medium.
Direct action: absorption of ionising radiation in a biological target.
Dual radiation action: a phenomenological theory of biological radiation damage with a contribution proportional to the dose and a contribution proportional to the square of the dose.
Electromagnetic radiation (EMR): A wave motion moving at the speed of light which transports transverse electric and magnetic fields oscillating at the wave frequency. Examples of EMR are radiowaves, microwaves, infrared, visible light, ultraviolet, x-rays, and gamma-waves.
Electron paramagnetic resonance (EPR): a technique for identifying free radicals based on the magnetic interactions of unpaired electrons with an external magnetic field.
Flash photolysis: an optical technique for identifying unstable reaction intermediates generated by a strong light pulse.
Fluence: The number of particles (particle fluence) or quantity of energy (energy fluence) incident on a surface from all directions divided by the area of that surface.
Fluence rate: fluence per unit time.
Fricke dosimeter: a radiation dosimeter based on the oxidation of aqueous ferrous ion to ferric ion in a strongly acidic solution.
G-unit: the number of species of a given type produced by an absorbed energy of 100 eV.
Gamma-rays (
g): electromagnetic radiation emitted by a radioactive nucleus.Graft co-polymerisation: covalent addition of a second monomer to an existing polymer induced by ionising radiation.
Gray (Gy): 1 gray equals 100 rad.
Gyromagnetic ratio: the ratio of the magnetic moment of a rotating charged object to its angular momentum. Also applies to the intrinsic angular momentum of elementary particles.
Half-life (T): time required for the decay of one-half of any starting number of radioactive atoms.
Homogeneous reaction kinetics: mathematical modeling of chemical reaction rates assuming that all species have a uniform spatial distribution.
Hydrated electron: a free electron in aqueous solution stabilized by dielectric interactions with the medium.
Hyperfine interaction: splitting of spectral lines induced by the intrinsic magnetic moments of nuclei.
Indirect action: absorption of ionising radiation in the medium within or external to biological targets.
Infratrack: narrow region near an ionising radiation track in which most of the energy is deposited.
Ionising radiation: typically refers to heavy charged particles, fast electrons, neutrons, x-rays, and gamma-rays.
Ion-pair: an entity generated by the interaction of ionising radiation with matter comprising an ion and its geminate fast electron.
Isobar: elements having the same mass number (A) and different atomic number (Z).
Isotope: elements having the same atomic number (Z) and different mass number (A).
Klystron: a linear diode device used to generate traveling microwaves.
LD50: the irradiation dose at which there is a 50% chance of lethality.
Large-target reaction kinetic: mathematical modeling of interactions between a small diffusive entity and a large biological target.
Linear accelerator (LINAC): a linear device in which bunches of electrons are accelerated by traveling microwaves.
Linear attenuation coefficient: t he local rate of particle loss or energy loss per unit length of track.
Linear energy transfer (LET): the energy per unit track length deposited by an ionising entity in a medium.
Mass number (A): the number of neutrons plus protons in an atomic nucleus.
Moderator: a light weight material used in used a nuclear reactor to slow down fast neutrons without absorbing them.
Molecular order: the number of atomic or molecule species that take part in a chemical reaction.
Molecular yield: hydrogen gas and hydrogen peroxide produced by radiolysis of water.
Neutrino: uncharged particles of zero rest mass and high energy which are emitted in beta decay.
Nuclear fission: splitting of a heavy element into several lighter elements induced by bombardment with neutrons or high-energy charged particles.
Nuclear magnetic resonance (NMR): a technique for determining molecular structures based on the interactions of magnetic nuclei with an external magnetic field.
Nuclear particles: typically refers to electrons, positrons, neutrons, protons, deuterons, and alpha-particles. More generally, entities present in cosmic rays and generated by high-energy accelerators.
Nucleon: a neutron or a proton in an atomic nucleus.
Ortho-hydrogen: see para-hydrogen.
Oscillator strength: a number related to the intrinsic probability of an optically-induced transition in an atom or molecule; also used for gamma-ray transitions.
Oxygen enhancement ratio (OER): the radiation sensitivity of a biological system in the absence of oxygen relative to that with oxygen
Pair-production: interaction of a photon with an atomic nucleus in which the photon is absorbed and an electron-positron pair is created.
Para-hydrogen: . In para-hydrogen the nuclear spins of the two protons are opposed. In ortho-hydrogen the nuclear spins are aligned. The two forms of molecular hydrogen have different physical properties and do not interconvert except under special conditions. For equilibrium at 0° C approximately 25% of the gas exists as para-hydrogen.
Photoelectric effect: interaction of a photon with an atom in which the photon is absorbed and an extra-nuclear electron is emitted. See Auger effect.
Photon: a hypothetical particle of zero rest mass moving with the speed of light and carrying electromagnetic momentum and energy. The photon energy (E) of monochromatic (single wavelength) EMR is related to the wavelength (l) by E = hc/l where h is Planck's constant (6.6262 x10-34 J-s) and c is the speed of light in a vacuum (2.9979 x 108 m/s).
Poisson distribution: a probability distribution which is applicable for a large number of equivalent trials with a low probability for success on a single trial .
Positron: a particle having the same mass and opposite charge as the electron.
Positonium: an unstable entity consisting a bound electron and positron.
Pulse radiolysis: an optical technique for identifying unstable reaction intermediates generated by a pulse of ionising radiation.
Quarks: the six building blocks of all elementary particles. The proton consists of two "up quarks" each with a charge of + 2/3 e and one "down quark" with a charge of - 1/3 e giving it a total charge of + e. The neutron is built from one "up quark" and two "down quarks", so that it has a net charge of zero.
Rad: 1 rad of any ionsing radiation corresponds to an energy absorption of 0.01 J/kg or 6.242 x 1013 eV/g.
Radiation sensitivity (S): the radiation dose which induces a specified change in a biological system; see D37.
Radiolysis: initiation of chemical reaction by exposure to ionising radiation.
Reaction order: the power dependence of reaction rate on the product of the reactant concentrations.
Relative biological effectiveness (RBE): the inverse of the radiation dose required for a specific response in a test system relative to the dose required to achieve the same response for 60Co gamma-rays (RBE = 1) under equivalent conditions.
Rem (roentgen–equivalent-man): the dose in rads multiplied by the relative biological effectiveness (RBE).
Repair: a process that converts potentially lethal radiation damage to a non-lethal state.
Roentgen (R): Exposure of dry air at 0° C and 760 torr to one roentgen of photon radiation produces ions of one sign carrying a total electrical charge of 2.58 x 10-4 C/kg of air when all secondary electrons are collected
Scavenger: a solute that removes free radicals from an irradiated system without generating reactive products.
Sievert (Sv): 1 sievert equals 100 rem.
Singlet molecular oxygen: a short-lived excited state of molecular oxygen ("active oxygen").
Spin: intrinsic angular momentum associated with fundamental particles, including the electron, positron, neutron, proton, other elementary particles, and some atomic nuclei.
Spur: a localized group of ion-pairs generated along the track of ionising radiation.
Stopping power : the same as linear energy transfer (LET).
Sub-lethal damage: radiation damage which does not induce an endpoint scored as lethality.
Survival curve: a graph of the logarithm of the fraction of undamaged entities vs the dose.
Synchrotron: a resonance device in which bunches of heavy charged particles are confined to a fixed ring orbit by a transverse magnetic field and accelerated by a variable frequency high-voltage field.
Target theory: a phenomenological theory of radiation damage in which the number of "hits" on a biological targets is related to the radiation dose.
Therapeutic ratio (TR): the ratio of the normal tissue tolerance dose for ionising radiation to the tumor lethal dose.
Track-segment model: a phenomenological theory that relates biological damage to linear energy transfer.
Transuranic elements: Artificial elements having atomic numbers higher than 92 created by bombarding relatively stable elements with high-energy particles, e.g., neptunium (Np93), plutonium (Pu94), americium (Am95), curium (Cm96), etc..
Tritium: the hydrogen isotope 3H1.
Tunneling: a quantum mechanical phenomenon by which a microscopic particle may pass through an potential barrier without loss of energy .
Van de Graaff generator: a linear device in which electrons or heavy charged particles are accelerated by a strong electrostatic field.
X-ray: electromagnetic radiation emitted when fast electrons strike a target.
Zeeman effect: shifts in optical spectra of atoms and molecules induced by an external magnetic field.
Acknowledgments
The cited figures are adapted from the following sources:
P. Alexander and J. T. Lett, Effects of ionizing radiations on biological molecules, In "Comprehensive Biochemistry, Volume 27 (Edited by M. Florkin and E. H. Stotz), Elsevier Publishing Company, New York, 1967.
H. Dertinger and H. Jung, "Molecular Radiation Biology", Springer-Verlag, New York, 1969.
J.H.O’Donnell and D.F. Sangster, "Principles of Radiation Chemistry", American Elsevier Publishing Company, New York, 1970.
L.I. Grossweiner, "The Science of Phototherapy", CRC Press, Boca Raton, 1994.
J.W.T. Spinks and R.J. Woods, "An Introduction to Radiation Chemistry", John Wiley & Sons, New York, 1976.
A.J. Swallow, "Radiation Chemistry", John Wiley & Sons, New York, 1973.
K.G. Zimmer, "Quantitative Radiation Biology", Hafner Publishing Company, New York, 1961.