Triplet state decay of xanthione (XT) and 4H-1-benzopyran-4-thione (BPT) in solid
b-cyclodextrin complexes Marek Sikorski a, b and Francis Wilkinson ba Faculty of Chemistry, University of A. Mickiewicz, 60 780 Poznan, Poland (e-mail: Sikorski@main.amu.edu.pl)
b Department of Chemistry, Loughborough University, Loughborough, Leicestershire, LE11 3TU, UK (e-mail: F.Wilkinson@lboro.ac.uk)
| Abstract | |
| Introduction | |
| Materials and Methods | |
| Results | |
| Discussion | |
| Conclusion | |
| References |
The formation of a b-CD inclusion complexes of xanthione (XT) and 4H-1-benzopyran-4-thione in the solid state has been reported. The results suggest that the solid state might be a particularly useful media for studying the photophysics of aromatic thioketones. In this paper we present results of studies concerning triplet state decay of xanthione and 4H-1-benzopyran-4-thione in solid b-cyclodextrin complexes. Time resolved spectra of phosphorescence and transient absorption spectra of XT/b-CD and BPT/b-CD solid complexes have been recorded. Complex kinetics of the decay have been often used to analyse such decays in solid systems. In contrast to the phosphorescence decay of XT/b-CD solid complex, the decay recorded at the maximum of T-T spectrum of XT/b-CD solid complex could not be fitted to the Albery model.
The photophysics and photochemistry of sulphur containing compounds has intrigued researchers for many years [1,2]. One of the reasons for such a growing interest is a considerable difference in the properties, both photophysical and photochemical, between sulphur containing compounds and their carbonyl analogues [3]. For example aromatic thioketones, owing to their unusual photophysical properties, have been the subject of increasing interest for researchers for at least 20 years. The growing interest in photochemistry of thioketones started from the first observation of the fluorescence from the S2 state [4,5]. It has also been reported that aromatic thioketones show room temperature phosphorescence from T1 state, quenching of the S2 and T1 states by molecules of the same compound in its ground-state, and photochemistry arising from both triplet and singlet states [1,2]. In many cases thioketones due to their absorption in the near UV and visible regions, high quantum yields of intersystem crossing and high quantum yields of singlet oxygen production can successfully replace ketones and make it easier to obtain information about biological systems [3]. Recently thioketones have been also described as useful probes in micellar studies [6].
A logical extension of the studies of thioketones in solution is to examine the effect upon the photochemistry of thioketones when placed in an environment where diffusional processes become restricted due the nature of the support material. It is interesting to learn more about the photochemistry and photophysics of cyclodextrin inclusion complexes of thioketones in the solid state. Cyclodextrins (CDs) are cyclic oligosacharides of six to eight a-D-glucose units (a-CD, six; b-CD, seven; g-CD, eight) endowed with internal hydrophobic cavities able to include a great variety of guest molecules. The schematic structure of cyclodextrins are presented in Figure 1.
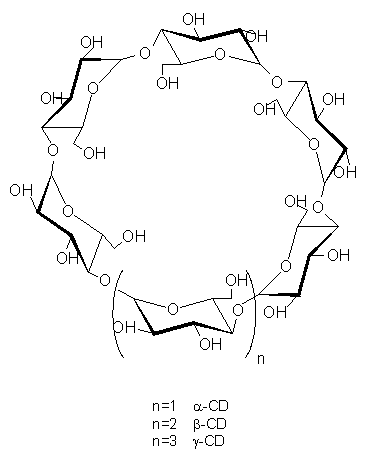
| Figure 1. A schematic presentation of cyclodextrins. |
The oligosacharide ring forms a torus with the primary hydroxyl groups of the glucose residues on the narrow end of the torus and the secondary ones on the wider end. All the hydroxyls are directed towards the outside of the cavity but they can also form hydrogen bonds with the guest included and/or also with external molecules.
It is well known that photochemistry and photophysics of molecules can be modified by inclusion in cyclodextrins [7]. These changes may be due to several effects. According to unusual properties, aromatic thioketones can constitute an excellent set of compounds to study the effect when molecules are inside a cyclodextrin cavity. Recently we have studied the effect of b -cyclodextrin and cellulose on the photophysical properties of some thioketones using diffuse reflectance laser flash photolysis, ground-state absorption and steady - state fluorescence methods [8]. The formation of a b-CD inclusion complexes of 4H-1-benzopyran-4-thione (BPT) in aqueous solution [9,10] and in the solid state [8] have been recently reported. The results suggest that the solid state might be a particularly useful media for studying the photophysics of aromatic thioketones 4H-1-benzopyran-4-thione and xanthione, whose structure is shown in Figure 2.
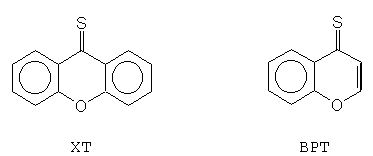
Figure 2. Structure and abbreviation for two thioketones studied - 4H-1-benzopyran-4-thione (BPT) and xanthione (XT).
Two studied thioketones are representatives of the group of aromatic thioketones. In this paper we present results of studies concerning triplet state decay of xanthione (XT) and 4H-1-benzopyran-4-thione (BPT) in solid b-cyclodextrin complexes. Complex kinetics of the decay have been often used to analyse such decays in solid systems. In this paper we present results on the triplet state decay of xanthione (XT) and 4H-1-benzopyran-4-thione (BPT) in solid b-cyclodextrin complexes. The study of such inclusion complexes of thioketones and the data on their triplet state decay in solids may bring new information about thioketones themselves and should increase the potential application of thioketones in studies of biologically important systems.
b-CD supplied by Aldrich was used as received. Although it is known that some commercial samples of cyclodextrins show impurity luminescence [11], no emission of b-CD was detected under our experimental conditions. The thioketones were synthesised and purified as described in Refs. [12,13]. Distilled water was used for preparing solutions.
CD solid complexes were prepared by dissolving a certain amount of the compounds in 0.011 M b-CD aqueous solutions under magnetic stirring at 70 0C for 4 hours. Water was then evaporated off in vacuo with a rotary evaporator and the solid obtained was dried under vacuum at 40 0C for 4 hours. Samples loading was around 1.1mg of substrate per g of b-CD.
Time resolved diffuse reflectance laser flash photolysis experiments were carried out by exciting samples at 354.7 nm in quartz cell. The laser was a HY hyper YAG 200 from Lumonics, the output from which was frequency tripled to give 22 mJ pulse with a pulse width of 8 ns. A 250 W Xenon lamp was used as monitoring source, and a R928 Hamamatsu photomultiplier was used as a detector. For transient emission data were acquired into a Tektronix 7612 D transient digitiser interfaced to a microcomputer. The details of this system and data processing can be found elsewhere [14,15].
The time resolved measurements in solutions were performed using a nanosecond laser flash photolysis system with the right angle geometry as described elsewhere [16,17]. The excitation source was a JK2000 Q-swiched Nd:YAG laser, the fundamental wavelength (1064 nm) of which was tripled to produce the harmonic at 354.7 nm
UV-VIS absorption spectra were recorded on a HP8453 diode array spectrophotometer. Ground sate diffuse reflectance absorption spectra were recorded on a PU8800 UV-VIS spectrophotometer equipped with an integrating sphere. Steady-state fluorescence spectra were recorded on a Fluoromax spectrofluorimeter (lexc = 355 nm). All the measurements were performed at room temperature for air-equilibrated samples.
A simple but widely adopted approach for describing the interaction of light with diffusing media has been formulated by Kubelka and Munk in 1931 [18]. In this formulation the remission function, F(R), for an ideal sample which is optically thick at the wavelength of choice and with a homogeneous distribution of absorbers throughout, is given by the Kubelka-Munk function, i.e.
![]() (1)
(1)
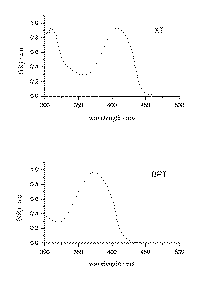
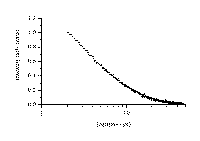
where R represents the observed diffuse reflectance from the surface of the sample, K and S represent absorption and scattering coefficients, respectively. It should be noted that values of F(R) are proportional to the absorber concentration. The diffuse reflectance absorption spectra of solid b -cyclodextrin complexes of BPT and XT were recorded and are presented in Figure 3.
Figure 3.
The ground state diffuse reflectance absorption spectra of xanthione b-CD and 4H-1-benzopyran-4-thione b-CD solid complexes plotted using the Kubelka-Munk remission function [14],[18].
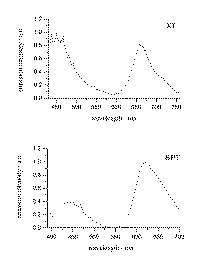
The emission spectra of XT/b-CD and BPT/b-CD solid complexes were also recorded and the corresponding spectra are presented in Figure 4.
The emission spectra of xanthione b-CD and
4H-1-benzopyran-4-thione b-CD solid complexes. The spectra are interpreted
as S2![]() S0 fluorescence and T1
S0 fluorescence and T1![]() S0 phosphorescence, (see text for explanation).
S0 phosphorescence, (see text for explanation).
The spectral properties of XT/b-CD and BPT/b-CD solid complexes are summarised in Table 1.
Table 1.- Spectral properties of xanthione b-CD and 4H-1-benzopyran-4-thione b-CD solid complexes at room temperature in air equilibrated samples (wavelength in nm).
Absorption lmax(S0® S2) |
Fluorescence lmax(S2® S0) |
Phosphorescence lmax(T1® S0) |
|
| BPT/b-CD solid complex | 374 |
439 |
618 |
| XT/b-CD solid complex | 405 |
453 |
657 |
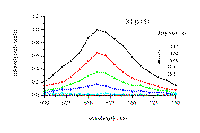
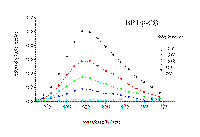
Laser excitation of cyclodextrin-thioketone solid complexes at 355 nm leads to an intense and readily detectable phosphorescence at room temperature. The phosphorescence decay of xanthione and 4H-1-benzopyran-4-thione in solid b-cyclodextrin complexes is particularly interesting. Time-resolved spectra of phosphorescence for both thioketones examined are presented in Figure 5 and 6. The respective delay times after the laser pulse are shown in the corresponding figures. The example decay traces recorded at the maxima of phosphorescence are also presented in Figure 5 and 6.
Figure 5
Figure 5. Time resolved spectra of phosphorescence of xanthione b-CD solid complex. The respective delay times are shown in the Figure. The excitation wavelength was 355 nm.
: a typical kinetic trace at 660 nm.
Figure 6.
Time resolved spectra of phosphorescence of 4H-1-benzopyran-4-thione b-CD solid complexes. The respective delay times are shown in the Figure. The excitation wavelength was 355 nm.
A typical kinetic trace at 620 nm.
In the XT/b-CD solid complex it
is interesting to compare the phosphorescence decay kinetics with the decay of the
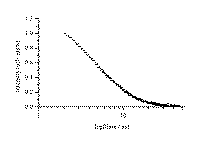
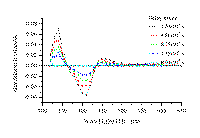
transient species observed. The transient spectrum of XT/b-CD solid complex is shown in Figure 7, at whose
bottom the transient spectrum of XT in acetonitrile is also given 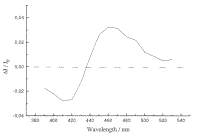
Figure 7. A. Transient absorption spectrum for xanthione b-CD solid inclusion complex observed upon 355 nm excitation.
Figure 7B.Transient absorption spectrum of xanthione observed upon 355 nm laser flash photolysis in deoxygenated acetonitrile.
Discussion
Ground state diffuse reflectance absorption spectra of xanthione b-CD and 4H-1-benzopyran-4-thione b-CD solid complexes presented in Figure 3 are similar to the absorption spectra of the corresponding thioketones found in solutions [1,2]. The maxima of diffuse reflectance absorption bands are 405 nm for XT/b-CD solid complex and 374 nm for BPT/b-CD solid complex. The spectral bands are interpreted as the S0® S2 bands by analogy to the situation for thioketones in solutions. For example in methanol the maximum of S0® S2 band for XT is at 405 nm and for BPT is at 374 nm.
Recently it has been reported that XT and BPT form inclusion complexes with cyclodextrin in aqueous solution [8,9,10]. The inclusion complexes of BPT have been clearly indicated by the spectral changes on addition of CD's to aqueous solutions of BPT. The linearity of Beneshi-Hildebrand plots reported by us previously is the evidence for the 1:1 stoichiometry of the complexes [9]. The binding constant for the BPT/b-CD complex has been reported as K = 950±90-1 [9].
It is apparent from the emission spectra presented in Figure 4, that for both thioketones examined two emissions bands are present in the emission spectrum. In general, aromatic thioketones show an intriguing luminescence behaviour including fluorescence from the S2 state, room temperature phosphorescence from the T1 state and thermally activated delayed fluorescence from the S1 state [2,16]. The spectra of emission XT/b-CD and BPT/b-CD solid complexes are interpreted as having either, the S2® S0 fluorescence and the T1® T0 phosphorescence. It is also possible that some contribution to the emission spectrum in the range attributed to phosphorescence is given by the thermally delayed fluorescence from the S1 state. Recently the energy gap between S1 and T1 states in 3-methylpentane solutions has been determined as 408 cm-1 for BPT and 618 cm-1 for XT [16]. It is expected a similar small energy gap between S1 and T1 sates also occurs in the case of thioketone/CD solid complexes. The small energy gap D (S1,T1) can suggest some contribution of the thermally delayed fluorescence also in the studied thioketone/b-CD solid complexes. Our recent observation for BPT and XT in solutions suggest that the thermally delayed fluorescence overlaps substantially in the spectral range where phosphorescence is observed for both these thioketones [16]. In our opinion, solid state might be a particularly useful media for studying the photophysics of aromatic thioketones 4H-1-benzopyran-4-thione and xanthione, either by observation of fluorescence or phosphorescence at room temperature.
Complex kinetics of the decay have been often used to analyse kinetics of decay in solid systems, for example the Albery model for dispersed kinetics in heterogeneous system have been successfully applied [19,20,21,22]. This model assumes a Gausian distribution of the logarithm of the rate constant about some mean value. According to the Albery model, the observed decay profile is composed of the summation of the contributions from each microscopic species and is given in the following form
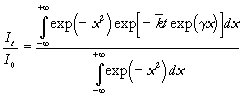 (2)
(2)
where
![]() (3)
(3)
It and I0
are the phosphorescence intensities after the pulse at time t and t = 0,
respectively, ![]() represents the average
rate constant, and g represents the width of the distribution. This
analysis leads to a conclusion that a plot of signal vs. log(time) should give a smooth
sigmoid curve, from which it should be possible to determine the most probable rate
constant as well as the with of the distribution. If g = 0, the Albery model becomes a simple
first-order exponential decay. The results obtained using the Albery model to analyse the
phosphorescence of the two thioketones examined in solid b -cyclodextrin complexes are presented in Table
2.
represents the average
rate constant, and g represents the width of the distribution. This
analysis leads to a conclusion that a plot of signal vs. log(time) should give a smooth
sigmoid curve, from which it should be possible to determine the most probable rate
constant as well as the with of the distribution. If g = 0, the Albery model becomes a simple
first-order exponential decay. The results obtained using the Albery model to analyse the
phosphorescence of the two thioketones examined in solid b -cyclodextrin complexes are presented in Table
2.
Table 2.- Kinetic data of xanthione b-CD and 4H-1-benzopyran-4-thione b-CD solid complexes at room temperature in air equilibrated samples [8].
Albery Model 1/ |
|
| BPT/b-CD solid complex | 2.61 1.11 |
| XT/b-CD solid complex | 2.24 0.62 |
The value of ![]() is a little higher for BPT/b-CD than for XT/b-CD solid complex. However, generally, it is
interesting to point out that the average rate constants,
is a little higher for BPT/b-CD than for XT/b-CD solid complex. However, generally, it is
interesting to point out that the average rate constants, ![]() , for both thioketones examined are similar to each other. What is
more interesting, the values of
, for both thioketones examined are similar to each other. What is
more interesting, the values of ![]() are
similar to the triplet state decay constants of these two thioketones in solutions at
infinite dilution, k0. It has been suggested that higher k0 values
in protic solvents when compared to non protic solvents indicate a significant
contribution of hydrogen abstraction to deactivation of thioketones in the triplet state
[23,24,25]. The quantum mechanical calculations also show that thioketones in their np* and pp* excited states are
capable of abstracting hydrogen from hydrogen donating media [26]. Bruhlmann and Huber
[23,25] suggested that higher k0 values of XT in protic solvent is not only due
to hydrogen abstraction but also to hydrogen bonding between the thioketone group and the
solvent. From this point of view, our data can be interpreted as indicating that probably
no specific interaction between thioketone and b-CD occurs.
are
similar to the triplet state decay constants of these two thioketones in solutions at
infinite dilution, k0. It has been suggested that higher k0 values
in protic solvents when compared to non protic solvents indicate a significant
contribution of hydrogen abstraction to deactivation of thioketones in the triplet state
[23,24,25]. The quantum mechanical calculations also show that thioketones in their np* and pp* excited states are
capable of abstracting hydrogen from hydrogen donating media [26]. Bruhlmann and Huber
[23,25] suggested that higher k0 values of XT in protic solvent is not only due
to hydrogen abstraction but also to hydrogen bonding between the thioketone group and the
solvent. From this point of view, our data can be interpreted as indicating that probably
no specific interaction between thioketone and b-CD occurs.
It has been shown that for BPT and XT, thermally activated delayed fluorescence from the S1 state is an important process in triplet state deactivation. From this point of view it is interesting to compare the lifetime of triplet state of XT and BPT at low temperature region. The lifetimes of the triplet state at 77 K was recorded as 44.6 ms and 63.0 ms for XT and BPT in 3-methylpentane, respectively [16]. Obviously at 77 K, the thermally activated delayed fluorescence from the S1 state of aromatic thioketones should be completely inhibited. The difference in the lifetimes of the triplet state between XT and BPT can be understood as a result of different energy gap between T1 and S0 states. However, it was also suggested that, in contrast to fluid solutions, triplet lifetimes recorded at 77 K in different matrixes are longer because the hydrogen abstraction is ineffective in the rigid glasses but also the low temperature can prevent the intramolecular decay path via a fast thermal equilibrium between the S1 and the T1 states. We can only suggest that for XT and BPT on solid supports used by us at room temperature the process of hydrogen abstraction is probably slow.
It is also interesting to note some
trend in changes of parameter g . It can be seen that the width of the
distribution in the case of BPT is nearly twice than for XT in b-CD solid complexes, suggesting that the
dispersion of ![]() is higher in the case of
BPT than XT. The higher value of parameter g for the BPT complex can be attributed to the
existence of two non interconverting major orientations of the BPT molecules within the b -CD cavity. By
contrast, XT can only be fitted within the cavity in one major orientation owing to its
symmetry. The analysis of the kinetics characterised in Table 2 would in principle require
understanding of the detailed physical and chemical interaction between molecules and
solid support, especially in the case of interactions which occur between b-CD and a guest
molecule. The reason for differences in g is not clear, however, the size of molecules and
the vibronic modes, different interaction with solid support can play some role. As
expected, a detailed discussion of phenomena which lead to such kinetics is not possible
with the data available at present. However, the dispersive kinetics should help us in
better understanding of the inclusion effect. To our best knowledge this is the first
report about photophysics of thioketones on surfaces.
is higher in the case of
BPT than XT. The higher value of parameter g for the BPT complex can be attributed to the
existence of two non interconverting major orientations of the BPT molecules within the b -CD cavity. By
contrast, XT can only be fitted within the cavity in one major orientation owing to its
symmetry. The analysis of the kinetics characterised in Table 2 would in principle require
understanding of the detailed physical and chemical interaction between molecules and
solid support, especially in the case of interactions which occur between b-CD and a guest
molecule. The reason for differences in g is not clear, however, the size of molecules and
the vibronic modes, different interaction with solid support can play some role. As
expected, a detailed discussion of phenomena which lead to such kinetics is not possible
with the data available at present. However, the dispersive kinetics should help us in
better understanding of the inclusion effect. To our best knowledge this is the first
report about photophysics of thioketones on surfaces.
Time-resolvent diffuse reflectance laser flash absorption spectra of XT/b-CD solid complex show a triplet-triplet absorption maximum at 460 nm. In general the spectrum is similar to those recorded by conventional method in solutions. For the sake of comparison, the transient absorption spectrum of XT in acetonitrile is also given. In the spectral region where the ground-state absorption of XT in acetonitrile dominates, negative absorbance changes due to the ground-state depletion constitute a mirror image of the absorption band-system due to the S0® S2 transition. Similar spectral and decay properties were found in the case of other thioketones [2,3,23,24]. The quenching of an excited triplet state molecule in solution by a molecule of the same compound but in the ground state, is an efficient way of triplet state decay for the aromatic thioketones. Values of the triplet lifetimes at infinite dilution recorded at room temperature are 3.0 ms and 2.4 ms for XT and BPT in acetonitrile solutions, respectively [23,24].
In contrast to the phosphorescence decay of XT/b-CD solid complexe, the decay recorded at the maximum of T-T spectrum of XT/b-CD solid complex could not be fitted to the Albery model. This indicates that the decay is more complex, probably some additional species, not only triplet state of XT, are responsible for a more complex decay. It is well know [27,28] that thioketones abstract hydrogens from suitable hydrogen donors and that CDs can act as hydrogen donors in solid inclusion complexes [7]. This can be the reason for such a complex decay in these XT/b-CD inclusion complexes. Transient decays did not vanish completely to the baseline indicating some small contribution from the long-lived transient species. Nevertheless, the existence of hydrogen abstraction processes between excited aromatic thioketones and b-CD in their inclusion complexes is unclear at present and requires further studies.
In this paper we present some from the photophysical properties of a cyclodextrin inclusion complexes of xanthione and 4H-1-benzopyran-4-thione in the solid state. This is to our best knowledge the first study of thioketones on solids when the time resolved diffuse reflectance laser flash photolysis method as well as ground-state absorption and steady-state fluorescence methods have been used.
The results presented describe the fluorescence from S2 state and phosphorescence from T1 state of thioketone/b-CD solid complexes. It has been shown that using surfaces instead of solutions we can prevent thioketones from very a efficient process of quenching the triplet state by a molecule of the same thioketone in its ground state. Also the quenching by oxygen does not play an important role when thioketones are placed on surfaces. The results suggest a different behaviour of thioketone/b-CD solid complex when compared to the situation when thioketones are placed in solution or rigid glass.
Acknowledgements
The author wish to thank The Royal Society/Central & East European Postdoctoral Fellowship and The Foreign & Commonwealth Office for a postdoctoral grant (to M.S.) and acknowledge the financial support of The British Council and KBN. Financial support under the joint research collaboration project from A. Mickiewicz University and University of Medical Sciences in Poznan is also gratefully acknowledged.
[1] R. P. Steer, Rev. Chem. Int., 1981, 4, 1.
[2] A. Maciejewski and R. P. Steer, Chem. Rev., 1993, 93, 67.
[3] R. S. Becker, S. Chakravorti, C. A. Gartner and M. de Graca Miguel, J. Chem. Soc. Faraday Trans., 1993, 89, 1007.
[4] J. P. Huber and M. Mahaney, Chem. Phys. Lett., 1975, 30, 410.
[5] M. H. Hui, P. De Mayo, R. Suau and W. R. Ware, Chem. Phys. Lett., 1975, 31, 257.
[6] A. Maciejewski, A. Jakubowska, E. Dutkiewicz and W. Augustyniak, J. Colloid Interface Sci., 1996, 177, 528.
[7] P. Bortolus and S. Monti, Advances in Photochemistry, 1996, 21, 1.
[8] M. Sikorski, M. Mir and F. Wilkinson, Chem. Commun., 1997, 395.
[9] M. Milewski, M. Sikorski, A. Maciejewski, M. Mir and F. Wilkinson, J. Chem. Soc. Faraday Trans., 1997, 93, 3029.
[10] M. Milewski, A. Maciejewski and W. Augustyniak, Chem. Phys. Lett., 1997, 272, 225.
[11] W. Chung, N. J. Turro, J. Silver and W. J. le Noble, J. Am. Chem. Soc., 1990, 112, 1202.
[12] A. Maciejewski, A. Safarzadeh-Amiri, R. E. Verrall and R. P. Steer, Chem. Phys., 1984, 87, 295.
[13] A. Maciejewski, D. R. Demmer, D. R. James, A. Safarzadeh-Amiri, R. E. Verrall and R. P. Steer, J. Am. Chem. Soc., 1985, 107, 2831.
[14] F. Wilkinson and G. Kelly, Handbook of Organic Photochemistry, ed. J. C. Scaiano, CRC Press, Boca Raton, FL, 1989, vol. 1, pp 293-314.
[15] F. Wilkinson, J. Chem. Soc., Faraday Trans II, 1986, 82, 2073.
[16] M. Sikorski, I. V. Khmelinskii, W. Augustyniak and F. Wilkinson, J. Chem. Soc., Faraday Trans., 1996, 92, 3487.
[17] F. Wilkinson, D.R. Worrall, D.J.McGarvey, A. Goodwin and A. Langley, J. Chem. Soc. Faraday Trans. 2. 1993, 89, 2385.
[18] P. Kubelka and F. Munk, Tech. Phys., 1931, 12, 593.
[19] W. J. Albery, P. N. Bartlett, C. P. Wilde and J. R. Darwent, J. Am. Chem. Soc., 1985, 107, 1854.
[20] Y. Mao, N. E. Breen and J. Thomas, J. Phys. Chem., 1995, 99, 9909.
[21] S. Pankasem and J. K. Thomas, J. Phys. Chem, 1991, 95, 6990.
[22] R. Krasnansky, K. Koike and J. K. Thomas, J. Phys. Chem., 1990, 94, 4521.
[23] U. Bruhlmann and J. R. Huber, Chem. Phys. Lett., 1978, 54, 606.
[24] M. Sikorski, W. Augustyniak, I. V. Khmelinski, V. V. Korolev and N. M. Bazhin, Chem. Phys. Lett., 1993, 209, 403.
[25] U. Bruhlmann and J. R. Huber, J. Photochem., 1979, 10, 205.
[26] K. Sumathi and A. K. Chandra, J. Photochem. Photobiol. A. Chem., 1988, 43, 313.
[27] D. R. Kemp and P. de Mayo, Chem. Commun,. 1972, 233.
[28] S. Formoshino, J. Chem. Soc. Faraday Trans. 2. 1976, 72, 1332.