| Photoiupac home page | Discussion | Photobiology.com home |
Self-assembly of large-scale aggregates of porphyrin from its dimers and their absorption and luminescence properties
Alexander V. Udalítsov1, Lev A. Kazarin2, Alexei A. Sweshnikov2
Faculty of Biology1, Faculty of Chemistry2, Lomonosov Moscow State University,
119899 Moscow, Russia; e-mail: avu@audaltsov.home.bio.msu.ru
Abstract
Protonated porphyrin dimers and possible structural formula of water-porphyrin dimeric complex were reported recently [1]. In this work properties of aggregates and protonated meso-tetraphenylporphine (TPP) dimers are investigated by absorption and luminescence spectroscopy and scanning electron microscopy. It is found that the absorption and fluorescence spectra obtained at a low and several times higher concentrations of porphyrin differ each other considerably. The changes in absorption spectra of TPP in the water-tetrahydrofuran-glycerol (83:7:10, v/v) mixture with time in the presence of 0.4 N HCl and the following appearance of the green precipitate in several days, indicate aggregation of the porphyrin. The near IR emission at 1000 nm, which is assigned to the fluorescence of donor-acceptor water-porphyrin dimeric complex, is revealed in the fluorescence spectra of TPP in aqueous tetrahydrofuran solution in the presence of 0.4 N HCl at a low concentration of porphyrin on excitation at 465 nm. In contrast, the IR emission is not observed in the solution with several times higher concentration of porphyrin, but a shoulder at ca. 800 nm is appreciable in the corresponding spectrum. The large-scale aggregates of TPP with the sizes approximately from 1 mm to several micrometers are found in thin films of the protonated porphyrin. It is proposed that the aggregates are formed in the result of self-assembly from different protonated porphyrin dimers and have a regulated inside structure.
Results

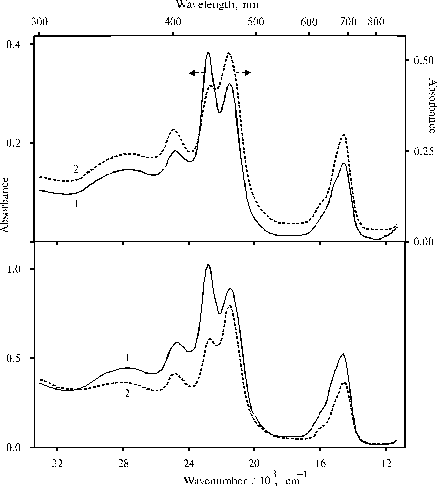
Fig. 1 Absorption spectra of TPP in water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid, panel A: at a low concentration of the porphyrin, 1 (optical pathway is 1 cm); and a medium concentration, 2 (optical pathway is 0.2 cm); and panel B: in water-glycerol-tetrahydrofuran solution (84 : 10 : 6, v/v) in the presence of 0.4 N hydrochloric acid, 1, and the same solution in 48 hour, 2.
Comments:
Changes of absorption spectra with a change of porphyrin concentration (panel A) and similar changes of the same solution but in the presence of glycerol with time (panel B) and the following in several days appearance of a slight green precipitate indicate aggregation of the porphyrin.
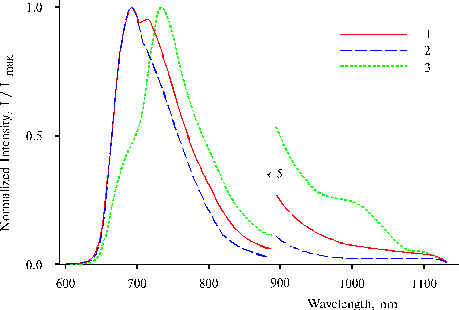
Fig. 2 Fluorescence spectra of TPP in water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid at a low concentration of the porphyrin on excitation at 403 nm, 1; 437 nm, 2; and 465 nm, 3. The spectra are normalized on the intensity of main emission band.
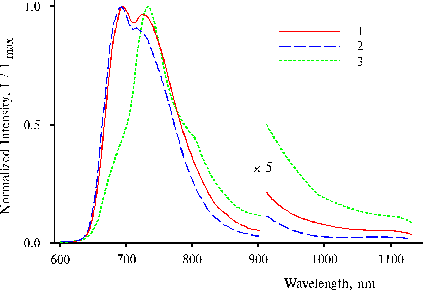
Fig. 3. Fluorescence spectra of TPP in water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid at a medium concentration of the porphyrin on excitation at 403 nm, 1; 437 nm, 2; and 465 nm, 3. The spectra are normalized on the intensity of main emission band.
Comments:
On excitation at 403 and 437 nm, two maxima or maximum and a shoulder of the main emission are observed at 693 and 730 nm in the fluorescence spectra (Fig. 3, curves 1 and 2), while the spectrum on the excitation at 465 nm exhibits only one maximum at 732 nm and two shoulders at 693 and ca. 800 nm (curve 3). The spectrum on the excitation at 465 nm (Fig. 2, curve 3) exhibits an appreciable shoulder of near IR emission at 1000 nm and maximum of the main emission at 735 nm. Similar IR emission has been found in solution and in thin films of TPP amino derivatives in the presence of water traces, and donor-acceptor complexes involving water are the radiative centers [2-3]. The maximum at 730-730 nm is suggested to be associated with the second intermediate state of mono-protonated dimer possessing trans NH configuration of the tautomeric isomer.
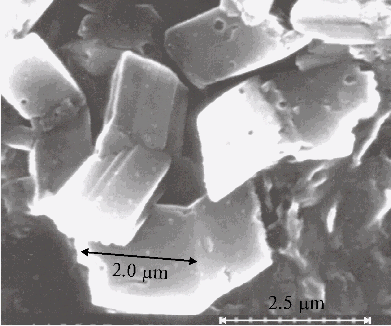
Fig. 4A. Microphotograph of TPP aggregates obtained on a glass plate directly after evaporation of water-tetrahydrofuran solution (93 : 7, v/v) in the presence of 0.4 N hydrochloric acid.
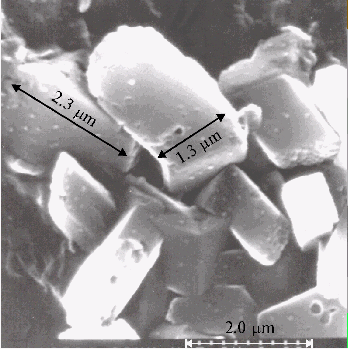
Fig. 4B. Microphotograph of TPP aggregates, the conditions are the same as in Fig. 4A.
Comments:
Large-scale aggregates formed from protonated TPP dimers are found in the thin films prepared on the glass plate. But no aggregates are observed by scanning electron microscopy in the absence of porphyrin in a solution.
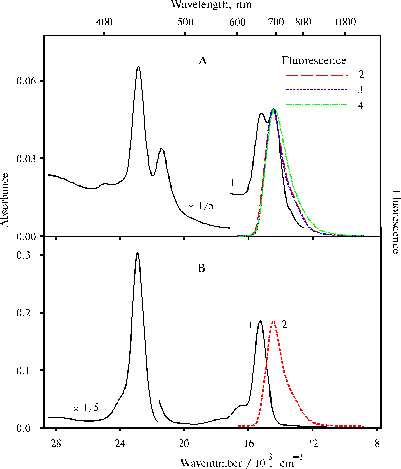
Fig. 5. Absorption (1) and fluorescence spectra (2-4) of TPP, panel A: in water-ethanol solution (91 : 9, v/v) in the presence of 0.4 N hydrochloric acid, 1; on excitation at 403 nm, 2; 437 nm, 3; and 465 nm, 4; panel B: in 50% aqueous solution of acetone (v/v) in the presence of 0.2 N hydrochloric acid, 1; on excitation at 437 nm, 2. The fluorescence spectra are normalized on the intensity of main emission band.
Comments:
No porphyrin aggregates were found in water-ethanol solution although similar TPP dimers are present according to the absorption spectrum. The fluorescence spectra of these dimeric forms under the same selective excitations (lex = 403, 437 and 465 nm) are very similar (panel A, curve 2-4). The fluorescence spectrum presented in panel B show that the band with the maximum of the main emission at 693 nm is the fluorescence of di-protonated TPP dimer. Hence, the fluorescence of TPP dimer with cis NH configuration of the tautomeric isomer (on excitation at 403 nm) is not observed in the spectra.
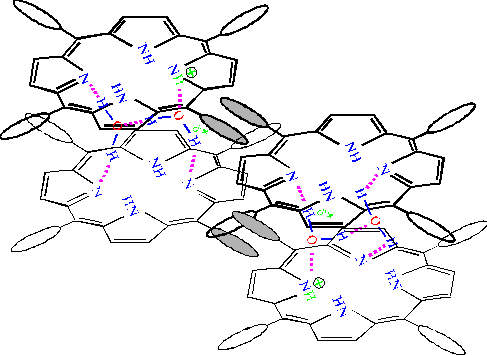
Fig. 6. The scheme proposed for the interaction between TPP dimers when the porphyrin dimers are assembled into the large-scale aggregates. The porphyrin molecules lying in the upper plane are depicted by thick formulas. Interacting phenyl rings of the porphyrins lying in the upper and lower planes respectively, are shaded in the figure. The distance between porphyrin macrocycles in the both dimers, where the energy of exciton interactions equals 1650 cm-1 [4], is estimated approximately 10 Å.
Comments:
It is proposed that self-assembly of porphyrin aggregates occurs from different protonated TPP dimers (trans and cis NH tautomeric isomers depicted on the left and right sides of the figure, respectively). The self-assembly proceeds in horizontal direction due to hydrophobic interaction between phenyl rings of the neighboring dimers and in vertical direction due to formation of hydrogen bonds between water molecules involved in the solvate covers of the dimers.
Conclusions
- the change of absorption spectra at two different concentrations of porphyrin and similar change of the spectra of analogous solution in the presence of glycerol with time indicate aggregation of the porphyrin in the solutions;
- a slow process of the aggregation leading to the appearance of a slight and fluffy green precipitate in the solution suggests some sort of self-assembly of the aggregates;
- the corresponding fluorescence spectra of similar two solutions with the different concentrations of porphyrin on excitation at 403 and 437 nm exhibit different intensity of the main emission at 730 nm. While on the excitation at 465 nm, the near IR emission at 1000 nm is revealed at a low concentration of porphyrin, but a shoulder at ca. 800 nm is appreciable in the spectrum of the solution with several times higher concentration of porphyrin;
- large-scale aggregates with the sizes approximately from 1 mm to several micrometers formed from protonated TPP dimers, are found in the thin films by scanning electron microscopy. The solid bars displayed in the microphotographs support the self-assembly of the aggregates. No aggregates was observed in the similar samples prepared without porphyrin;
- these aggregates are thought to be form in the result of self-assembly from different protonated porphyrin dimers due to hydrophobic interaction between phenyl rings of neighboring porphyrin dimers and hydrogen bonds between water molecules involved in water -porphyrin dimeric complex.
References
[1] A.V. Udalítsov, A.A. Churin, Internet Photochem. and Photobiol., (1998) http://www.photobiology.com/IUPAC98/Udaltsov/index.htm
[2] A.V. Udal'tsov, J.Photochem. and Photobiol. B: Biol., 37 (1997) 31-39.
[3] A.V. Udal'tsov, L.A. Kazarin, Biochemistry (Moscow), 61 (1996) 367-373.
[4] A.V. Udalítsov, Biochemistry (Moscow), 62 (1997) 1026-1033.