| Photoiupac home page | Discussion | Photobiology.com home |
DIASTEREOSELECTIVE PHOTOSENSITIZED RADICAL ADDITION TO FUMARIC ACID DERIVATIVES BEARING OXAZOLIDINE AUXILIARY GROUPS
Gigliola Campari, Raffaella Mosca, Maurizio Fagnoni, Mariella Mella, Angelo Albini
Department of Organic Chemistry, University of Pavia, Via Taramelli 10, 27100 Pavia, Italy
e-mail: fagnoni@chifis.unipv.it fax: +39-0382507323
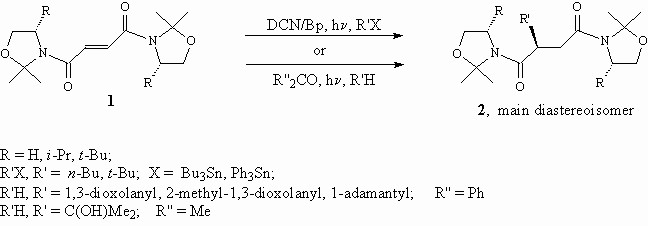
Radical addition to olefins has become a synthetic useful process in organic synthesis particularly when the reaction occurs with a high degree of diastereoselectivity. Only a few cases of stereoselective radical addition to a stereogenic centre on an acyclic alkene bearing a chiral auxiliary have been reported in the literature.

The diastereoselection in the formation of the 2-alkyl succinic acid derivatives 2 ranged from 88.9 to > 98.5%. Addition of the adamantyl radical only occurred from the bridgehead positon.
We can conclude that the mild conditions of photochemical radical generation allow for optimal exploitment of chiral amides for diastereoselective alkylation. Furthermore, the resulting 2-substituted chirals succinic acid derivatives 2 can be elaborated as suitable building blocks for organic synthesis.