| Photoiupac home page | Discussion | Photobiology.com home |
FLUORESCENCE EMISSION STUDIES OF AN AZLACTONE DERIVATIVE IN POLYMER FILMS; AN OPTICAL SENSOR IN pH MEASUREMENTS
Canan Karapirea, Sıddık İçlia, Serap Alpb, Kadriye Ertekina, Berrin Yenigüla and Emür Hendena
a
Department of Chemistry, Faculty of Science, Ege University, Bornova, 35100 Izmir, Turkeyb
Department of Chemistry, Faculty of Education, Dokuz Eylül University, Buca, Izmir, Turkey
Abstract
Azlactone molecules can be protonated in acidic media, and the process of protonation is irreversible due to ring opening in solutions. A new fully reversible pH sensor has been developed 4-(p-N,N-dimethylamino phenyl methylene)-2- phenyl-5-oxazolone (DPO), an azlactone derivative, embedded in a plasticized PVC polymer film. The sensor membrane is completely transparent and exhibits pH induced colour change which is capable of measuring of pH in the range of 1-7 and has a pKa value of 3.4. In agreement with crystal phase studies, DPO displays enhanced fluorescence emission quantum yield, Qf = 0.52, and fluorescence emission lifetime, t f = 1.35 ns, in immobilized PVC film matrix, compared to Qf @ 0.03 and t f 0.02-0.03 ns in solutions. Stokes shift decreased to 46 nm in plastisized PVC film, with respect to Stokes shift of 81 nm in acetonitrile solution. Singlet energy, Es=60.4 kcal/mol, of DPO in PVC film, which is 1.1-1.4 kcal/mol lower with respect to solutions, is taken as evidence of lessened singlet-triplet crossings in excited state of DPO in immobilized phase, and result in enchanced fluorescence emissions.
Key words ¾ ¾ Optical sensor, fluorescence emission, azlactone, pH, polymer film.
Extensive studies on 4-arylene-2-aryl-5-oxazolones (azlactones) [ 1-5] proved that in liquid state;
photooxydations and at chemical electron transfer reactions,
but in solid state;
i) molecular structure is photostable in solid state and fluorescence emission enhanced
(Qf » 10-1-1.0).
ii) favored photophysical and photochemical properties in solid state, resulted in use of azlactones at semiconductor devices, at electrophotographic photoreceptors and at non-linear optical materials.
Under the light of literature, one would study the fluorescence emission of azlactone molecule in an immobilized phase, where higher yield of fluorescence emission and improved stability toward chemical and photochemical environment could be reached. Our earlier studies revealed that 4-(p-N,N-dimethylbenzal)-2-phenyl-2-oxazolone, DPO, gives better fluorescence quantum yields, Qf, in solution and in solid state in comparison to other benzal oxazolones. In fact in solid state Qf is about unity. The colour of DPO is bright red in crystal form, in contrast to pale yellow-orange colours of other azlactone crystals. Long wavelength absorption of DPO (465 nm) in liquid phase, may display a colour change in polymer film if protonated in an acidic medium, which may be used as a practical visual observation.

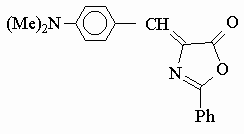
DPO
Because of the presence of carbonyl oxygen and nitrogen atom in hetero ring of azlactones, protonation would occur in acidic medium and would alter the absorption bands. p -Bonds of hetero atoms would open in protonated forms of azlactone, that would result in loss of absorption at longest wavelength band. Oxazolone ring opens in liquid state in acidic medium, but in immobilized phase of polymer film hetero ring is expected to be stable and one may be able to achieve reversible process of protonation. Then one may be able to study pH dependent absorption and fluorescence emission spectra of azlactones.
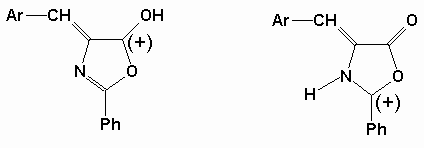
Glass pH electrodes are the most widely used instruments in all kinds of sciences including chemical, biochemical and environmental. But glass electrodes show poor performance at low (0-2) and high (12-14) pH values. In order to overcome this handicap numerous chemical sensor systems have been developed in recent years [ 6-10] . The determination of pH with fluorescence based optosensing techniquies have advantages over methods based on spectrophotometric sensing owing to the sensitivity of fluorescence. Wolfbeis et al. [ 11] have used certain coumarins and trisodium salt of 8-hydroxy-1,3,6-pyrenetrisulfonate (HTPS) for measuring near neutral pH values. Zhujun and Seitz [ 9] prepared a pH sensor by electrostatic immobilization of HPTS on an anion exchange membrane. This sensor allowed the measurements of pH in the range of 6 to 8. Kawabata et al. immobilized a monolayer of fluorescein directly to surface of a modified optical fiber [ 12] . Jordan and Walt [ 13] developed an energy transfer based sensor using eosine and phenol red combination for physiological ranges. Werner and Wolfbeis presented a pH sensitive membrane for the pH 10-13 range. They covalently immobilized the N8 dye to cellulose on a polyester support. They measuared the pH dependent absorption of the membrane at 555 nm via optical fibers [ 14] . Wolfbeis reports quenching effect of widely used 2-nitrophenyl octyl ether plasticizer on fluorescence emission of porphyrines in plasticized polymer films [ 15] .
The azlactone derivative DPO contains available active centers for proton attacks and it is appropriate for use as a optode pH sensor.
2. Experimental details
2.1. Materials
4-(p-N,N-dimethylbenzal)-2-phenyl-2-oxazolone (DPO) was synthesized and purified as described [ 5a] . The membrane components, PVC ( high molecular weight ) and the plasticizers bis-(2-ethylhexyl)phtalate (DOP), bis-(2-ethylhexyl)sebecate (DOS) and 2-nitrophenyl octyl ether (NPOE) were supplied from Fluka, bis-(2-ethylhexyl)adipate (DOA), the lipophilic anionic additive potassium tetrakis-(4-chlorophenyl) borate (PTCPB) and tetrahydrofuran (THF) were obtained from Aldrich, the acids used (HNO3 and HCl) and the regeneration buffer (titrisol, pH= 7 ±0.02) were from Merck. Studies performed in buffered solutions of acids were carried out with N,N-bis(2-hydroxyethyl)-2-amino-ethanesulfonic acid (BES) from Sigma. Acid solutions and buffers used, for investigation of pH effects, were prepared with a high quality pure water. Pure water was obtained from an apparatus working with reverse osmosis principle from Elga. All the other chemicals were obtained from Fluka and Merck, and were used as supplied. The organic solvents used (acetonitrile, chloroform, THF) were all of spectrophotometric grade, and were used as supplied. The polyester support (Mylar type) was provided from Du Pont, Switzerland.
2.2. Polymer film preparation
The optode polymer films were prepared from a mixture of 120 mg of PVC, 240 mg of plasticizer, equimolar PTCPB to dye DPO and 1,5 ml of THF (dry). The concentration of DPO in mixture was in the range of 10-3 - 10-4 M (about 20 mmole dye/kg polymer). Fluorescence emission of DPO was lost at lower than 10-4 M concentrations, in plasticisized PVC. The resulting cocktails were spread onto a 125 m m polyester support (Mylar type) , by means of a spreading device (from Heidelberg- Germany). PVC films were kept in a THF containing dessicator and in the dark for the photostability of the membrane and the damage from the ambient air of laboratory was avoided. Each sensor PVC film was cut with a width of 120 mm and located diagonally into the sample cuvette. The advantage of this kind of a placement was to improve the reproducibility of the measurements. The films were photographed under Leitz ortopcan polarise microscope, connected to a computer and thicknesses were measured in reference to thickness of Mylar type polyester support. Figure 1 shows the picture of film contain DPO in plasticisized PVC matrix, where polyester support and plasticisized PVC film is visible. PVC films were measured to be in the range of 5-10 m m.
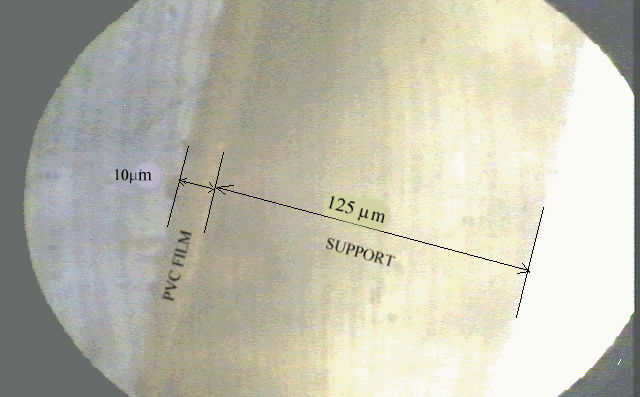
Fig. 1. Picture of DPO in plastisized PVC matrix, on Mylar polyester support.
2.3. Spectroscopic measurements
The absorption spectra of the polymer films were measured at Jasko V-530 UV-VIS spectrophotometer, the fluorescence emission spectra was recorded at PTI-QM1 fluorescence spectrophotometer. pH measurements were performed with a pH meter Jenway 3040 Ion Analyser calibrated with Merck pH standarts of pH 7,00 (titrisol buffer) and 4 at 20oC. Fluorescence quantum yield of DPO was measured in reference to absorption and fluoresence emission of n-dodecyl perylene diimide, l exc= 485 nm. Absorption and fluorescence emission spectra of polymer films were measured in quartz UV cells, which were filled with water, and polymer films were placed in diagonal positions. For pH induced measurements, neutral water was replaced with buffered (with BES) or unbuffered solutions at various pHs. Preference of BES is due to its adoptance to human serums.
3. Results and discussion
3.1. UV-VIS and Fluorescence Emission Spectroscopy Studies
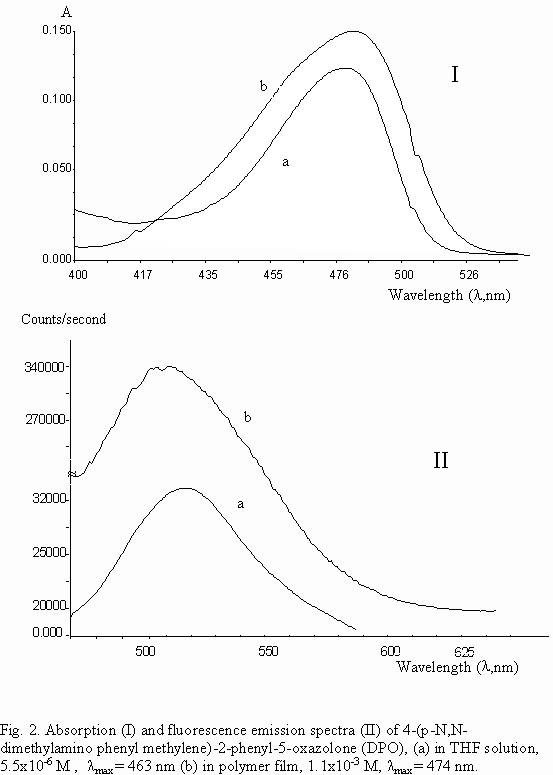
Absorption and fluorescence emissions of DPO both in THF solution and in plastisized PVC film had shown similar spectra (Fig. 2 and Table 1). Wavelentgh of absorption, l max, in film is seen to be shifted about 10 nm compared to absorption l max in THF solution. Blue shift of absorption of DPO may be related to enchanced conjugation in immobilized polymer phase by hindrance of vibrational rotational motions. A decrease of Stokes shifts(D l ), in the order of acetonitril, THF solutions and PVC film, 82, 61 and 46 nm, respectively is evident. Difference between acetonitrile and THF solutions can be related to differences in solvent polarities, but the decrease of Stokes shift in polymer film would arise from lowered difference of equilibrium geometries between ground and excited states in immobilized phase respect to solutions.
Table 1. UV-VIS Spectroscopy data (l , nm, and e , L.mol-1.cm-1), Stokes shifts (D l , nm), fluorescence quantum yield,Qf, radiative lifetime, t o(ns)*, fluorescence lifetime, t f(ns)# , fluorescence rate constant, kf(109 s-1)§ , and singlet energy, Es(kcal/mol) of synthesized DPO in solutions and in PVC polymer film.
t o = 3.5x108/n 2max.e max. D n 1/2 , n max:wavenumber in cm-1, D n 1/2: the half width of the selected absorption in wavenumber units of cm-1.
# t
f = t o.Qf§
kf = 1/t oIn agreement with crystal phase studies[ 5b] , fluorescence quantum yield of DPO in polymer film matrix, Qf=0.52, has increased about twohundredfold, respect to Qf of DPO in solutions, and fluorescence lifetime of DPO in polymer film matrix, t f=1.35 ns, has increased about fiftyfold, with respect to t f of DPO in solutions. These data can be taken as proofs that azlactone molecule DPO fluoresces better in immobilized PVC matrix. Singlet energy of DPO in polymer film matrix, Es=60.4 kcal/mol, is observed to be lowered about 1.1-1.4 kcal/mol, with respect to Es of DPO in solutions. This result may be taken as an evidence of lessened singlet-triplet intersystem crossings of azlactones in polymer film matrix, with respect to in solutions, in support of enhanced fluorescence emission. Induced steric effect in
immobilized phase, with respect to solvated molecule in solution, may endorse enhancement fluorescence emission. Azlactone molecule is expected to lie in planar form in polymer film matrix, as suggested for in crystalline structure [ 5b] .
3.2. Studies in polymer film matrices
3.2.a. Choice of indicator
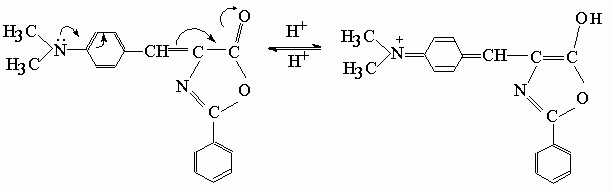
4-(p-N,N-dimethylbenzal)-2-phenyl-2-oxazolone, DPO, was selected among the oxazolone derivatives, because of its longest wavelength absorption (l max= 465 nm) in visible region, and highest fluorescence quantum yield (Qf @ 0.003, Table 1) compared to all of the azlactones studied earlier[ 5] . Also it is observed that the dye DPO has an excellent solubility in plasticized PVC matrix. High molar extinction coefficient (e = 2-5x104 M-1cm-1 in liquid phase) of DPO is another parameter of advantage of selection in polymer film matrices[ 6] . UV absorptions of DPO were recorded in acetone and dimethylformamide solutions, and l max @ 465 nm absorption was found to be unchanged on titration with perchloric acid dissolved in glacial acetic acid. But when DPO solution was titrated with tetrabutylammoniumhydroxide solution in 2-propanol, it was found that l max @ 465 nm absorption disappeared and an intense band appeared at l max @ 360 nm, and a colour change from orange to yellow occurred. Acidification with glacial acetic acid, did not changed the colour either. In general it appears that non-aqueous solutions of DPO is in part stable in acidic medium, but unstable in basic medium. This result may indicate that DPO is protonated in acidic medium as shown in the scheme below. Probably resonance contribution from methylated aniline ring contributes to the formation of enol form in hetero ring.
3.2.b. Choice of polymer and plasticizer
Our experiments with PVC and ethyl cellulose proved that PVC matrices give better and faster responses to both absorbance and fluoresence emission measurements at various pH values with DPO. A comperative study on the effect of different plasticizers and matrix materials on the performance of pH sensor also is performed. The polymer film matrix components were mixed according to the amounts given in table 2. Fluorescence emission spectra in 10-8 to 10-1 M HCl solutions, were recorded for each six compositions above. It was found that the best resolved emission spectra, relative signal change, fast response time and good reproducibility were obtained with DOP plasticizer in composition of PF-1.
Figure 3 shows the resolution differences of DPO fluorescence emission in DOP (PF-1) and in DOA(PF-2) plastisizers, in PVC polymer film matrices. As seen fluorescence emission resolution is lost in DPO/DOA(PF-2) composition, from neutral to acidic solutions.
3.2.c. pKa value of DPO
pKa value of DPO were calculated in polymer film matrices. UV absorption of DPO were recorded in buffered, BES/HCl solutions, and in unbuffered, HCl solutions, (Fig. 4). The buffer capacity of the sample matrix is seen to influence the sensor response strongly. Normalized absorption parameters(Ax/Ao) are calculated by division of measured absorption to absorption at neutral pH as Ao (pH @ 7). Figure 5 shows the plot of normalized absorbance, Ax/Ao versus to measured pH values in buffered solutions. pKa value was found as pKa = 3.4, pH value at half of the normalized absorption. pKa was also calculated by using non-linear fitting algorithm of Gauss-Newton-Marquardt method [ 14] .
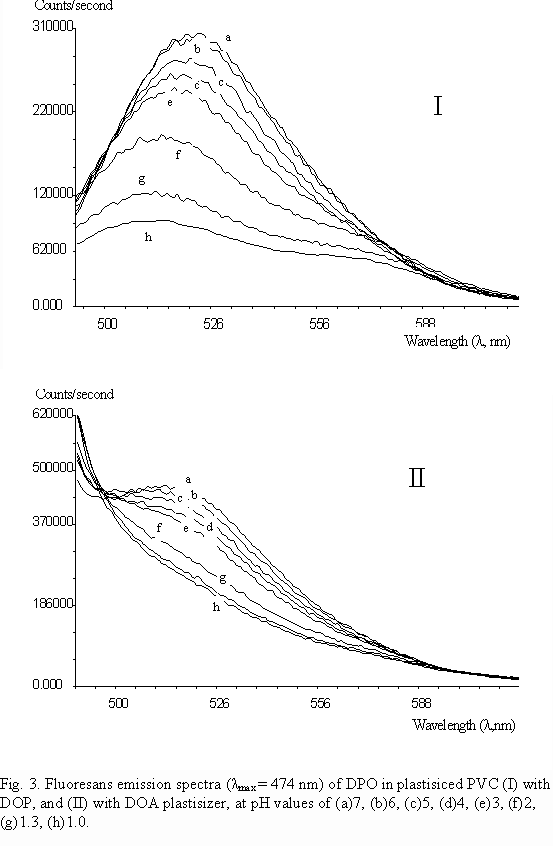
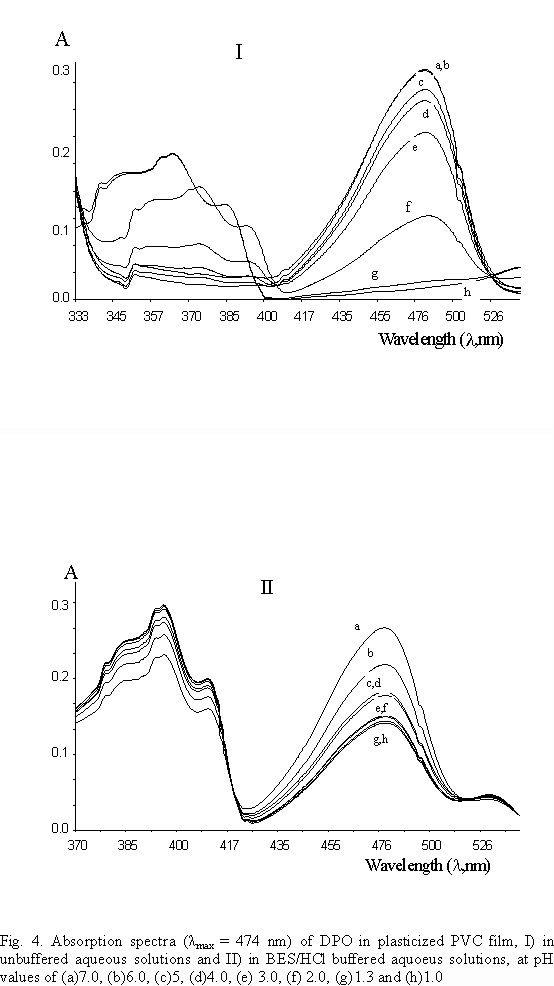
Table 2. Polymer film matrices of DPO prepared with plasticers of DOP, DOA, DOS, NPOE in polymers of PVC and ethyl cellulose.
|
|
Polymer (mg) |
Solvent (ml) |
Plasticizer (mg) |
Anionic Lipophilic Aditive |
Dye |
Notes |
|
PF-1 |
PVC 120 |
THF 1,5 |
DOP 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Transparent l max 478nm |
|
PF-2 |
PVC 120 |
THF 1,5 |
DOA 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Transparent l max 474nm |
|
PF-3 |
PVC 120 |
THF 1,5 |
DOS 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Transparent l max 480nm |
|
PF-4 |
PVC 120 |
THF 1,5 |
NPOE 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Transparent l max 480nm |
|
PF-5 |
Ethyl Cellulose120 |
80:20 Toluene/ Ethanol |
DOP 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Turbid |
|
PF-6 |
Ethyl Cellulose120 |
80:20 Toluene/ Ethanol |
DOA 240 |
PTCPB 20mmol/kg polymer |
DPO 20mmol/kg polymer |
Transparent l max 480nm |
pKa = pH + log(Ax – Ab)/( Aa – Ax) Aa and Ab: absorbances of acidic and basic forms.
Ax: absorbance at a pH near to the pKa.
In accordance with direct measurement, pKa = 3.42 value was calculated from above equation.
This result points that DPO may be an effective pH sensor in acidic region of pH 1-7.

Fig.5. Plot of pH versus to normalized absorbance of DPO in plasticized PVC film.
3.2.d. DPO as an optode pH sensor
DPO in composition of PF-1 polymer matrix was found to give re-producible results on fluorescence emission measurements when pH was varied from 7 to 1 and from 1 to 7. Dynamic pH range of 7 to 1 corresponds to HCl concentrations of 10-8 M to 10-1 M, respectively. The detection limit was observed to be 10-9 M HCl concentration. It was also observed that the colour of the polymer film was orange at pH 7, but gradually changed to pink as it reached to pH 1, and reverse colour change was observed opposite pH change. Proton binding was seen to be faster than that of unbinding. The regenaration time of DPO pH sensor was found to be 30 minutes in H2PO4-/HPO42- buffer solution, that gives a pH of 7 ± 0.02.
Plot in figure 6 outlines the pH induced absorbance variation versus time. Absorbance of DPO is found to decrease(or drift) about 2% after third cycle.
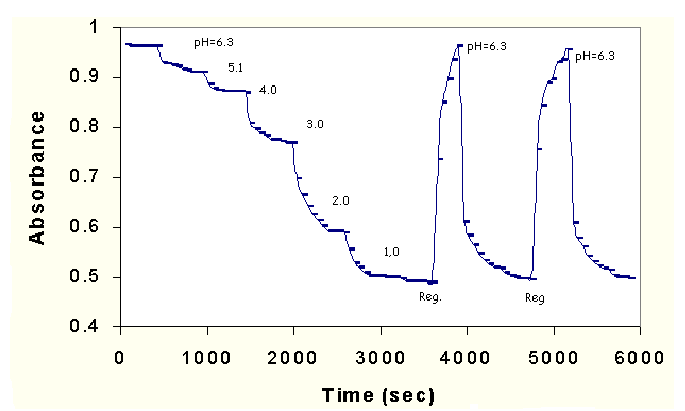
Fig.6. Plot of pH induced absorbance of DPO in plasticized PVC film versus time
4. Conclusion
It is proven that azlactone molecules would exhibit much higher fluorescence emissions in immmobilized polymer film matrices with respect to solutions, studied for 4-(p-N,N-dimethylbenzal)-2-phenyl-2-oxazolone, DPO, molecule. pKa value of DPO in PVC matrix, calculated from absorption spectra, is found to be 3.4, pointed a protonation process in acidic medium. Completely reversible protonation-deprotonation of plastisized PVC film of azlactone DPO, detected both in fluorescence emission and absorption spectra, allowed us to develop an optode pH sensor of pH 1-7 range. The optode pH sensor of DPO is found to reach 90% of the signal intensity in less than 5 minutes (t 90). High performance with respect to response time, reproducibility,, dynamic pH range of 1-7, and the visible colour change are the main advantageous parameters for this new optical sensor of pH measurements.
Acknowledgements
We thank to project support funds of Research Center of Ege University (EBILTEM), to State Planning Organization of Turkey (DPT), to Scientific Research Council of Turkey (TUBITAK) and to Alexander von Humboldt Foundation of Germany.
References
[ 1] M. Muneer, R.K. Tikare, P.V. Kamat and M.V. George, Can. J. Chem. 65 (1987) 1624.
[ 2] V.M. Dixit, V. Bhat, A.M. Trozzolo and M.V. George, J. Org. Chem. 44 (1979) 4169.
[ 3] a) E.F. Ullman and N. Baumann, J. Am. Chem. Soc. 92(20) (1970) 5892.
b) Von N. Baumann, Chimia 27 (1973) 471.
[ 4] a) B.M. Krasovitskii and B.M. Bolotin, in Organic Luminescent Materials, VCH
Verlags.mbH, Weinheim, pp 144-146, 1988.
b) B.M. Krasovitskii, M.B. Stryukov, V.Ya. Simkin, I.V. Lysova, V.I. Minkin, S.E. Kovalev
and L. Sh. Afanasiadi, Iz. Akad. Nauk SSSR Seri Fiz.44(4) (1980) 812.
[ 5] a) S. Icli, H. Icil, S. Alp and H. Koc, Spectrosc. Lett. 27(9) (1994) 1115.
Lett. 32(4) (1999) 553.
[ 6] O.S. Wolfbeis, in Fiber optic chemical sensors and biosensors, Vol. I, CRC Press, London, pp
304-310, 1991.
[ 7] L.A. Saari and W.R. Seitz, Anal. Chem., 54 (1982) 821.
[ 8] G.F. Kirkbrigth, R. Narayanaswamy, and N.A. Welti, Analyst, 109 (1984) 1025.
[ 9] Z. Zhujun and W.R. Seitz, Anal.Chim. Acta, 160 (1984) 47.
[ 10] H. Offenbacher, O.S. Wolfbeis, and E. Fürlinger, Sensors and Actuators, 9 (1986) 73.
[ 11] O.S. Wolfbeis, E. Fürlinger, H. Kroneis, and H. Marsoner, Fresenius Z. Anal. Chem. 314
(1983) 119.
[ 12] Y. Kawabata, K. Tsuchida, T. Imasaka, and N. Ishibashi, Anal. Sci:, 3, (1987) 7.
[ 13] D.M. Jordan and D.R. Walt, Anal. Chem., 59 (1987) 437.
[ 14] T.Werner and O.S.Wolfbeis., Fresenius J Anal. Chem., 346 (1993) 564.
[ 15 ] D.B. Papkovsky, G.J. Mohr and O.S. Wolfbeis, Anal. Chim. Acta, 337 (1997) 201.