| Photoiupac home page | Discussion | Photobiology.com home |
PORPHYRIN SENSITIZERS FOR BIOMEDICAL APPLICATIONS
Rodica -Mariana ION
ZECASIN S.A.,Photochemistry Dept.,Splaiul Independentei 202,Bucharest-79611, Romania; E-mail: irma@pcnet.ro.
ABSTRACT
The porphyrins represent very chemically versatile compounds. The treatment of tumor with porphyrins and red light, led to rapid necrosis of tumor tissue which was the result of the destruction of the tumor microvasculature.
The purpose of this paper is to summarize the knowledge on the photophysical and photochemical properties of some porphyrinic photosensitizers - 5,10,15,20-tetra-(1-phenyl)-porphyrin (TPP), 5,10,15,20-tetra-(1-naphthyl)-porphyrin (TNP) and 5,10,15,20-tetra-(p-sulfonato-phenyl)-porphyrin (TSPP) in order to evaluate the main factors affecting the efficiency as photosensitizers [solvent effect (binary mixture water + DMSO), aggregation and ionization processes effect] for some biological systems (blood human cells, human brain cells). The absorption, the fluorescence and the cytofluorimetry, have been used in this paper as the main spectral methods. Monomer-dimer and J-aggregation, have been evaluated.
The non-sulfonated and the sulfonated porphyrin types, are much better incorporated into cells in aggregated forms than in monomeric forms, (at the non-cytotoxic DMSO concentration in water - 0.5%-), the first being able to penetrate more rapidly the cellular membrane than the second.
Tested in vitro on brain tumors, with a pulsed nitrogen laser with emission at 337 nm, the best photosensitizer was TNP. Since the vasculature is the site the most affected by PDT, the inhibition of the mitogenic stimulation of the human blood cells by the porphyrins photosensitization, was also took into account.
Keywords: porphyrins, photodynamic therapy, biomedical applications, brain tumor.
1. INTRODUCTION
Photodynamic therapy (PDT) of cancer is a more selective approach for the treatment of different malignancies compared with other standard available treatment modalities. A sensitizer, light, and oxygen are used to cause photochemically induced cellís death [1].
The sensitizer should comply with some necessary conditions:
- be able to be incorporated into malignant cells with a much greater efficiency than be incorporated into normal cells (selective incorporation in tumor tissue) [2];
- be able to fluoresce efficiently and produce singlet oxygen, or other species which can destroy malignant cells [3];
- be non-toxic for healthy cells and, if possible, it should be quickly expelled from the organism;
-be a pure compound [4];
-be activated with UV, visible and near-IR emitting light [5];
- be soluble in the bodyís tissue fluids so that it can be injected and transported efficiently to the tumor site.
Porphyrins are promising candidates for sensitizing PDT action, because they follow all the above mentioned necessary conditions [6].
Tumor differ in a number range from most normal tissues, which is particularly the case of the malignant tumor cells, because the malignant tumor cells have some cell surface antigens that are different from those of normal cells. Monoclonal antibodies directed towards tumor cell antigens can be covalently coupled to photosensitizing dyes [7].
The purpose of this paper is to summarize some personal data concerning the porphyrins applications on the brain tumors treated photodynamically. The solvent effect (binary mixture water + DMSO), the aggregation and ionization processes effects for some biological systems (blood human cells, human brain cells) are discussed by means of the absorption, the fluorescence and the cytofluorimetry.
2. EXPERIMENTAL PART
Type of photosensitizers
5, 10,15, 20 -tetra-(1-phenyl) -porphyrin (TPP), 5, 10,15, 20 -tetra-(1-naphthyl)-porphyrin (TNP), 5, 10,15, 20 -tetra- (p-sulfonato-phenyl) -porphyrin (TSPP), were synthesized and purrified in tha laboratory after the literature methods [7-9].
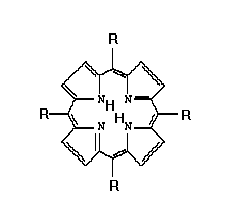
All of them were solubilized in DMSO-Water (0.5%-99.5%) binary mixture of solvents. Their chemical structure are shown in Fig. 1 and Tab. 1.

Fig.1. The structure of the studied porphyrins
Tab. 1. The substituents for used porphyrins
============================================================
Substituent(R) Name of the porphyrin
============================================================
C6H5 - 5, 10,15,20 -tetra-phenyl -porphyrin (TPP)
C10 H8 - 5,10,15,20 -tetra-1-naphthyl-porphyrin (TNP)
C6H4-SO3 - 5, 10,15, 20 -tetra- p-sulfonato-phenyl -porphyrin (TSPP)
=============================================================
Methods and Apparatus
Absorption spectra
The absorption spectra were measured with a spectrometer SPECORD M400, Carl Zeiss Jena, with double beam and microprocessor.
Fluorescence spectra
Fluorescence spectra were measured with an Aminco-Bowmann spectrofluorimeter. The range of the concentration from 0.09 m g/ml to 9 mg/ml for fluorescence and absorption spectral measurements were used.
Flow cytometry measurements
The 3-fluorescence flow cytometer (Cytron Absolute, Ortho) equipped with an argon ion laser was used and with a peak fitting program using Gauss type functions on a PC computer program. Was appreciated the number of stained cells (lymphocytes and granulocytes), incubated with porphyrins.
Optical Microscopy
Video recordings of the vascular effects for cells during the light irradiation, were obtained by means of a Leitz microscope and a Leitz Aristoplan microscope, as already reported [5].
Sample prelevation
We have made in vitro irradiation experiments of tumoral tissues obtained from different types of cerebral tumors prelevated from 10 patients on whom there were made previously surgical interventions. The samples were obtained by cutting pieces from tumor tissues. An aseptically excised brain tissue was cut on a sterile paraffin plate into pieces about 1 mm of diameter. These fragments were washed in MEM and then were places into wells. MEM with 10% FCS in total volume of 0.15 ml was added into each wells. The sample was irradiated by laser after 24-48 h incubation time. The dye impregnation was done immediately after operation.
Experimental irradiation procedure
We have chosen these dyes due to their absorption is strong along a large spectral range either, which made it is possible to excite their fluorescence at 337.1 nm, the wavelength of the nitrogen pulsed laser radiation. (fig.2).
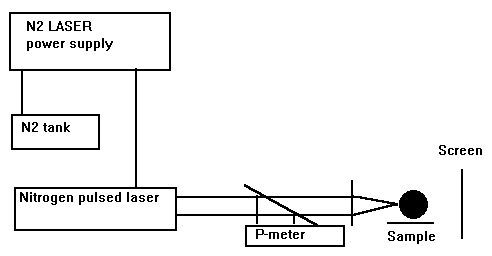
Fig.2. Experimental set-up for tumor irradiation (in vitro) (from Ref.[10])
The radiation of the nitrogen pulsed laser is emitted at 337.1 nm with a power density ranging from 1 to 3.5 mW/cm2, which correspond to a frequency range 3-10 pulses/sec.
Determination of the tumor volume from digitized computed tomography scans
Target volumes were defined on computed tomography (CT) scans which were digitized with an automatic laser scanner. The stored images were transferred to a personal computer. The macroscopic tumor shape was defined in every CT slice (slice thickness 5 to 8 mm) using a drawing tool. The number of pixels n enclosed by this contour was determined with a custom shaped image processing program.
3. RESULTS AND DISCUSSION
In solvents like DMSO, the porphyrins have strong absorption bands in visible region of spectra between 500 and 700 nm and a broad absorption band in the range 300-350 nm (Soret band) (Tab.2).
Tab.2. Spectral properties of PDT dyes
=============================================================
Sensitizer Solvent Absorption bands (nm)
-------------------------------------------------------------------------------------------------------------
TPP (1) 360 415 515 550 591 646
TPP (2) 362 412 437 646 705
TNP (1) 355 414 514 552 590 645
TNP (2) 357 412 434 650 707
TSPP (1) 355 414 518 545 595 648
TSPP (2) 360 345 420 525 675 710
==============================================================
As a result of water addition in case of non-sulfonated porphyrins, the changes characteristic for the aggregation of dye are seen. The Soret band became broader blue shifted and splitted as a consequence of a mixture existence of a face-to-face and oblique geometry, and the Qx band is red shifted. It is characteristic probably for the formation of large oligomers, because in a case of dimerization the molar absorption coefficient is decreasing, not increasing as we observed in our case [11,12], Tab.3.
Tab.3. The porphyrinic forms in DMSO:Water binary mixture of solvents
=====================================================
Solvent Non-sulfonated Sulfonated
DMSO:H2O(%) porphyrins porphyrins
------------------------------------------------------------------------------------------------
100:0-80:20 anionic+dimeric forms neutral+ anionic forms
80:20-50:50 dimeric forms dimeric forms
50:50-25:75 cationic+aggregated forms neutral forms
50:50-37:63 - monocationic forms
25:75-0.5:99.5 aggregated (J)+monomeric dicationic+aggregated forms forms
Even with only one emission band (695 nm for TPP, 692 nm for TNP and 685 nm for TSPP), the intensities of the fluorescence of these porphyrins in 0.5% DMSO in water (lexc= 337.1 nm) are 6 times to 10 times lower than in 100% DMSO. It shows again that the sulfonated and non-sulfonated porphyrins in aqueous DMSO solution mixture exist like a monomeric and dicationic- aggregated structures mixture (J-aggregates) with emission properties comparable with those of a monomer.
The advantage of exciting the photosensitizers in UV is that the available sources in that range are more powerful than those with emission in red and we could obtain a more efficient effect for PDT. One has be outlined that the use of UV radiation for PDT experiments is recommended for the tumor bed treatment following the surgical extraction of the tumor tissue, Tab.4.
Tab. 4. The PDT (in vitro) experimental conditions (From Ref.[16]).
==============================================================
Patient Sensitizer/ Irradiation conditions Incubation Tumor type
Solvent time dose power density time
(h) (J/cm2) (mW/cm2) (h)
A TSPP 4 14.3 1.05 24
0.1 mg/ml(1) Parasagial meningiom
-----------------------------------------------------------------------------------------------------------------
B TSPP 1 6.3 1.75 1
0.1 mg/ml (2) Right temporal tumor
---------------------------------------------------------------------------------------------------------------
C TPP 2 12.6 1.75 2
0.1 mg/ml (1) Left parentheral tumor
---------------------------------------------------------------------------------------------------------------
D TPP 1 6.3 1.75 24 Left frontal tumor
0.05 mg/ml (2)
---------------------------------------------------------------------------------------------------------------
E TNP 3 19 1.75 48 Malignant meningioma
0.1 mg/ml (1)
-----------------------------------------------------------------------------------------------------------------
F TNP 3 19 1.75 48 Intraventricular tumor
0.1 mg/ml (2)
==============================================================
(1) DMSO; (2) 0.5% DMSO in water.
The area Ai of the i-th slices for each tumor sample, was determined as
Ai= Pixel size (length) x Pixel size (width ) x n (1)
The pixel size was determined using the scaling.
The determined tumor area of each slice was multiplied with itís slice thickness di. The total tumor volume V (method I) was approximated by eq, 2
m
V tumor (cm3)= S Ai (mm2) x di (mm) /1000 (2)
i=1
where : m is the number of discrete tumor area.
The method II for tumor volume involved in PDT was agreed with the hemiellipsoid model (eq.3).
1 4 p 1 W
V = --- x ----- x ( --- x -----) x h (3)
2 3 2 2
where: l is length; W is width; h is heigth. The obtained results are shown in Tab.5.
Tab.5. The experimental responses from the studied tumors incubated with porphyrins
==============================================================
Tumor response TPP TNP TSPP
--------------------------------------------------------------------------------------------------------------
TTV (%) 32.9 99.8 30.8
(Total tumor
volume after PDT)
(Method I)
(Method II) 31.76 95.34 25.6
The non-sulphonated porphyrins are better sensitizers (F1O2= 0.84 (TPP) and 0.94(TNP)) than the same compounds but sulfonated (0.78) [11].
The efficiency of the incorporation of these dyes into human blood cells changed in the same manner as the singlet oxygen generation [2,6,8]. The sulfonated porphyrins were better incorporated into leukocytes than into granulocytes (contrary to the non-sulphonated ones)[13-15]. It can suggest that aggregated forms (J-aggregates) generated for the porphyrins in DMSO:Water are better penetrating the membranes but in the membrane dyes are deaggregated by the interaction with lipids and then exhibit photodynamic efficiencies as the monomeric form incorporated from DMSO. The penetration of these dyes depends on the type of cells being usually higher for the granulocytes than for the lymphocytes, Tab.6.
Tab.6. Incorporation of dyes into cells.
=====================================================
Dye Cells Solvent % stained Mean intensity Dye state
cells of fluorescence
------------------------------------------------------------------------------------------------
TPP L 1 1.6 87 M
G 1 12 120 M
L 2 4.5 82.3 A
G 2 63.8 113.1 A
TNP L 1 4.7 86.3 M
G 1 36.1 123 M
L 2 4.5 69 A
G 2 63.8 92 A
TSPP L 1 44 89.7 M
G 1 67 121 M
L 2 0 0 M+A
G 2 0.1 0 M+A
=======================================================
L=lymphocytes; G=granulocytes; 1=100% DMSO; 2=0.5% DMSO in water;
M=monomer; A+aggregated.
The studied porphyrins were tested into PDT application on the brain tumoral cells provided from human (in vitro), the PDT protocol yielding to the disappearance of the tumor. Tested in vitro on brain tumors, with a pulsed nitrogen laser with emission at 337.1 nm, the best photosensitizer was TNP [16]. The effect of PDT on the microvasculature of the brain tumor cells in the first few hours after treatment was studied by optical microscopy. The light led to a rapid necrosis of tumor which was not the result of the direct killing of tumor cells, but destruction of tumor microvasculature. It has been shown that the vascular damage expressed by a decreased blood flow stasis are immediate and major consequences of the photodynamic treatment with photodynamic systems introduced in these experiments.
The first observable signs of the destruction occur in the collagen fibers and other connective tissue elements located in the subendothelial zone of the tumour capillary wall. The altered permeability and transport through the endothelial cell layer resulting from erythrocyte swelling and increased intraluminal pressure may be another keys feature in the photodynamic destruction.
From the micrograms recorded before and after the PDT-treatment of the brain culture tissue, could be observed the disappearance of the tumor zone from the studied cellular mass [10]. Since the vasculature is the site the most affected by PDT, the inhibition of the mitogenic stimulation of the human blood lymphocytes by the dyes photosensitization was also took into account.
4.CONCLUSIONS
The non-sulphonated porphyrins are better sensitizers than the same compounds but sulfonated. The efficiency of the incorporation of these dyes into human blood cells changed in the same manner as the singlet oxygen generation: the non-sulfonated porphyrins were more efficiently incorporated than the sulphonated porphyrins and they are more efficiently incorporated into leukocytes than into granulocytes. The aggregated forms (J-aggregates) of porphyrins generated in DMSO:Water binary mixture favor the penetration of the membranes. Tested in vitro on brain tumors, with a pulsed nitrogen laser with emission at 337.1 nm, the best photosensitizer was TNP. Since the vasculature is the site the most affected by PDT, the inhibition of the mitogenic stimulation of the human blood cells by the porphyrins photosensitization, was also took into account.
5.REFERENCES
1. R.M.Ion, The photodynamic therapy of cancer -a photosensitization or a photocatalytic process?,Progr.Catal.,1,55 (1997);
6. D.Frackowiak, A.Planner, R.M.Ion, K.Wiktorowicz, Incorporation of
dyes in resting and stimulated leukocytes,in "Synthesis, properties and applications of near-infrared dyes in high technology fields", Ed.S.Daehne, Kluwer Acad.Publ., NATO ASI Series,1998
8. A.Planner, R.M.Ion, K.Wictorowicz, D.Frackowiak, The incorporation of porphyrins in human leucocytes measured by flow cytometry absorption and emission spectroscopy, "First Internet Conference on Photochem. Photobiol.", http://www.netsci-journal.com/97v3/intro.htm.
11. R.M.Ion, Spectral studies of TSPP and TSNP used in PDT.I.Monomer-dimer equilibrium, Rom. J. Biophys., 6(3-4), 213-218 (1996);
12. R.M.Ion, The photophysical properties of some porphyrins in binary mixtures of solvents,Rom.J.Biophys.,2,(1998);
13. R.Carter,E.W.Meyer,Introduction to principles of flow cytometry, in "Flow cytometry.A practical approach",M.G.Ed.Oxford Univ.Press,Oxford-New York-Tokio,1990,p.1-28;
14. R.M.Ion, Spectral analysis of the porphyrin into human blood cells, J.Biomed.Opt.,4(3),319(1999);
15. R.M.Ion, M.Grigorescu, F.Scarlat, V.I.R.Niculescu, K.Gunaydin, Light, electron and photons beam effects on TSPP used in PDT, J.Balkan Union Oncology, 3(2)000(2000);
16. M.L.Pascu, L.Danaila, A.Popescu, M.Pascu, R.M.Ion, Researches concerning the application of laser photodynamic therapy in neurosurgery, Rom.Reports Phys., 2(1999);