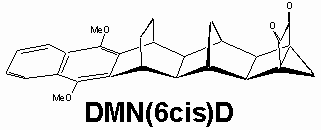
 • in this work we have investigated a series of novel dimethoxynaphthalene (DMN) and dimethoxybenzene (DMB)-bridge-dione compounds of the type:
• in this work we have investigated a series of novel dimethoxynaphthalene (DMN) and dimethoxybenzene (DMB)-bridge-dione compounds of the type:| Photoiupac home page | Discussion | Photobiology.com home |
Ultrafast Intramolecular Electronic Excitation Energy Transfer in Rigidly Bridged Systems
K.P.Ghiggino, T.A.Smith,
N.Lokan & M.N.Paddon-RowSchool of Chemistry, University of Melbourne, Parkville, Australia, 3010.
School of Chemistry, University of New South Wales, Sydney, Australia, 2052
Introduction
•
• in rigid donor-{saturated hydrocarbon bridge}-acceptor (D-B-A) systems, triplet and singlet EET can take place over substantial distances by a type of electron exchange mechanism involving through-bond (TB) coupling with the bridge orbitals.1,2
•
for the TB mechanism, the EET rates (kEET) are expected to display a strong exponential distance dependence of the form:k
EET = A.exp(-bn)where n is the number of bonds in the bridge relay, and
b is an attenuation coefficient.•
experimentally determined values for b lie in the range of 2 - 2.5 per bond.Compounds
 • in this work we have investigated a series of novel dimethoxynaphthalene (DMN) and dimethoxybenzene (DMB)-bridge-dione compounds of the type:
• in this work we have investigated a series of novel dimethoxynaphthalene (DMN) and dimethoxybenzene (DMB)-bridge-dione compounds of the type:
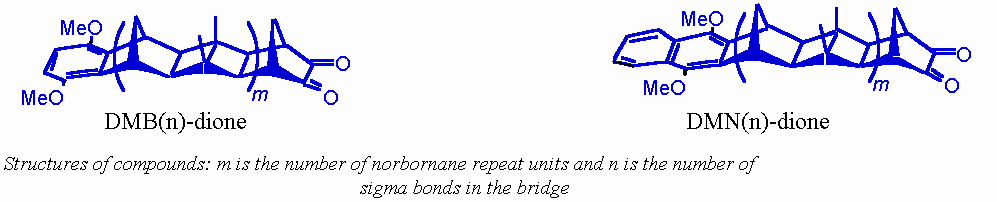
•
these studies can be compared to those carried out on comparable molecules containing a ketone group rather than the dione,1 and less rigidly-linked molecules containing similar chromophores.3
Steady-State Fluorescence Spectroscopy
•
Significant quenching of DMN/DMB emission (relative to the relevant model compound) is observed in all the molecules in the DMN/DMB(n)-dione series (Figure 1). Emission from the dione moiety is also evident (see inset) despite the absorption from the dione at the excitation wavelength (295 nm) being negligible (e ≈ 15 M-1cm-1).
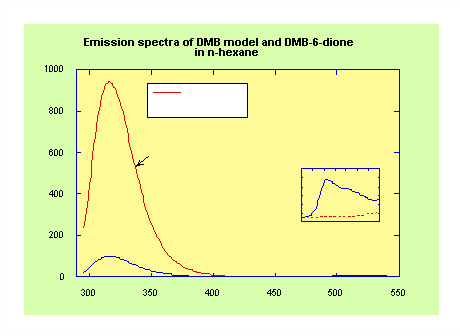
•
The excitation spectrum monitoring in the dione region (505 nm) shows that the majority of this emission is due to absorption in the aromatic region (295 nm, Figure 2a).• These results suggest that efficient EET is taking place.
• The amount of quenching indicated understates the true amount of energy transfer in these molecules since severe photodegradation of the dione group occurs (Figure 2).
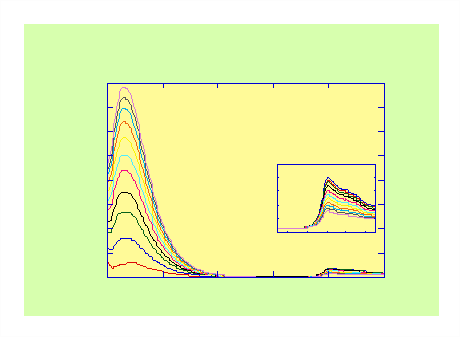
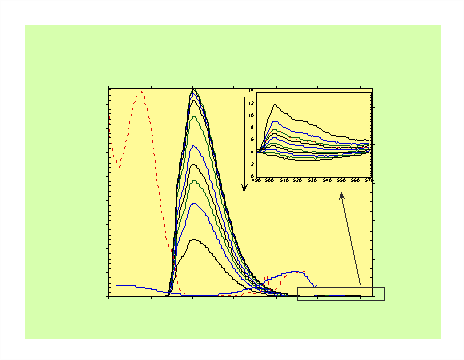
Figure 2. Fluorescence spectra of DMN(10)D and DMB(6)D in n-hexane as a function of irradiation time showing photodegradation of dione groups.
•
This photodegradation artificially increases the emission from the aromatic groups at the expense of the dione since the energy transfer pathway is removed following photodegradation.Time-Resolved Fluorescence Measurements
•
Time-resolved measurements provide a method of determining the rate of EET. These methods are also independent of the problems associated with the absolute fluorescence intensities encountered in the steady-state measurements due to the photodegradation.• The fluorescence decays monitoring in the aromatic region are complex requiring multi-exponential functions for adequate fits and extracting rates is therefore difficult.
• Monitoring the emission in the dione region provides a more reliable measure of the extent of EET, since the rate of formation of the dione emission should be independent of the photodegradation process.
• The rise and decay of the fluorescence from the dione region of the DMN(10)D is shown in Figure 3. An upper limit on the rise time is estimated to be ~40ps (with a decay time of ~11.6 ns corresponding to the dione decay). This leads to an estimated rate of energy transfer of ~2.5x1010 s-1. The equivalent rate for DMN(6)D is estimated to be >1011 s-1.
Figure 2. Fluorescence 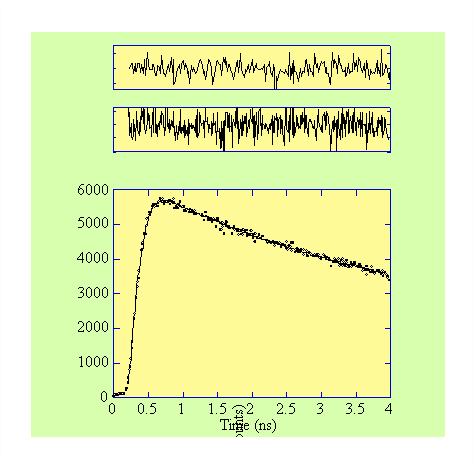 rise and decay profile from DMN(10)D in n-hexane (
rise and decay profile from DMN(10)D in n-hexane (
Conclusions
•
the rate of EET appears to be several orders of magnitude faster in DMN(10)D than predicted by through space mechanisms.4•
the rate of EET in DMN(6)D was beyond our present time resolution but appears to be at least three orders of magnitude faster than for the comparable DMN(6)-ketone molecule.1•
these results suggest that efficient TB EET is occurring in these molecules and that this is more efficient than in the corresponding ketone molecules1 and other comparable, flexibly linked systems3 which have similar donor and acceptor groups.• the results indicate the importance of the linking bridge in determining the mechanism and rate of energy transfer processes.
References
1 (a) Oevering, H., Verhoeven, J. W., Paddon-Row, M. N., Cotsaris, E. and Hush, N. S. Chem. Phys. Lett., 1988, 143, 488-495. (b) Kroon, J., Oliver, A. M., Paddon-Row, M. N. and Verhoeven, J. W. J. Am. Chem. Soc, 1990, 112, 4868-4873.
(2) (a) Closs, G. L., Piotrowiak, P., MacInnis, J. M. and Fleming, G. R. J. Am. Chem. Soc., 1988, 110, 2652-2653. (b) Closs, G. L., Johnson, M. D., Miller, J. R. and Piotrowiak, P. J. Am. Chem. Soc., 1989, 111, 3751-3753.
(3) (a) Hassoon, S., Lustig, H., Rubin, M. B. and Speiser, S. J. Phys. Chem., 1984, 88, 6367-6374. (b) Levy, S.-T., Rubin, M. B. and Speiser, S. J. Am. Chem.
Soc., 1992, 114, (c) Toledano, E., Rubin, M. B. and Speiser, S. J. Photochem. Photobiol. A: Chem., 1996, 94, 93-100.(4) Lokan, N., Paddon-Row, M.N., Smith, T.A., La Rosa, M., Ghiggino, K.P. and Speiser, S., J. Am. Chem. Soc., 1999, 121, 2917-2918.