| Photoiupac home page | Discussion | Photobiology.com home |
LSER AND TLSER ANALYSIS OF SOLVENT EFFECT ON THE SENSITIZED PHOTOOXYGENATION OF LIDOCAINE.
A. L. Zanocco,* E. Lemp M., N. Pizarro U.,
J. R. de la Fuente and G. Günther S.
Universidad de Chile, Facultad de Ciencias Químicas y Farmacéuticas Departamento de Química Orgánica y Fisicoquímica
Casilla 233, Santiago - 1, Santiago, Chile, FAX: 56 - 2 - 6782878, e-mail:
azanocco@ll.ciq.uchile.clINTRODUCTION
Lidocaine, 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide, is a local anaesthetic that reduces ventricular arrhythmia associated with myocardial infarction, myocardial infarct size and ischemic myocardial injury (1). The protective effects of lidocaine have been attributed to its membrane stabilizing properties, by acting as a short-lived free radical scavenger. Since lidocaine plays a protective role in ischemic myocardial damage (2) and has been used in cardioplegic solutions its antioxidant properties have been investigated. Das and Misra (3) using EPR, found that lidocaine is a powerful hydroxyl radical scavenger. Furthermore, these authors propose that lidocaine is a powerful scavenger of singlet oxygen. Also, they found that lidocaine was more effective than b -carotene, sodium azide and histidine in quenching singlet oxygen. However, there are no kinetic data accounting for its reactivity towards singlet oxygen in the different microenvironments present in biological systems, and no data regarding reaction mechanism.
In this work we report kinetic results obtained on the sensitized photo-oxidation of lidocaine using both steady state and time-resolved methods. Additionally, LSER and TLSER correlations were employed to analyze kinetic solvent effects and explain differences between lidocaine reactivity and that of a typical aliphatic amine. The acetamido group present in lidocaine (Fig. 1) is strongly electron withdrawing, which will affect the reactivity of the diethylamino group towards singlet oxygen, and these groups may interact intramoleculary, e.g., by hydrogen bonding, which will be solvent dependent.

Figure 1. Structure of lidocaine
EXPERIMENTAL
Chemical reaction rate constants were determined by using a 10 ml double wall cell, light-protected by black paint. A centred window allows irradiation with light of a certain wavelength by using Schott cut-off filters. Circulating water maintained the cell temperature at 22 ± 0.5 °C. Rose Bengal (l max = 557 nm) was the sensitizer. Illumination was with a visible, 200 W, Par lamp. In these experiments lidocaine consumption was monitored by using a Hewlett Packard 5890 gas chromatograph equipped with a NPD detector.
Steady state competitive experiments were performed in a cell allowing irradiation with light of a selected wavelength by using a Schott cut-off filter. The temperature was 22 ± 0.5 °C. In apolar solvents, Rubrene (l max = 520 nm) was the sensitizer. In polar solvents or in buffered solutions (pH = 5 and 10) Rose Bengal (l max = 557 nm) was the sensitizer. Illumination was with a visible, 150 W, Par lamp.
Time resolved phosphorescence measurements were carried out in 1 cm path fluorescence cuvettes. Phenazine excitation was by absorption of the third harmonic (355 nm, ca. 15 mJ per pulse) of the 6-ns light pulse of a Quantel Brilliant Q-Switched Nd:YAG laser. When TPP or Rose Bengal was the sensitizer, excitation was by absorption of the 500-ps light pulse of a PTI model PL-202 dye laser (419 nm or 556 nm, ca. 200 m J per pulse). A PTI model PL-2300 nitrogen laser was used to pump the dye laser. A liquid N2 cooled North Coast model EO-817P germanium photodiode detector with a built-in preamplifier was used to detect infrared radiation from the cuvette. The detector was coupled to the cuvette at a right-angle. The only elements between the cuvette face and the diode cover plate were an interference filter (1270 nm, Spectrogon US, Inc.) and a cut-off filter (995 nm, Andover Corp.). The preamplifier output was fed into the 1 MW input of a digitizing oscilloscope Hewlett Packard model 54540 A. Computerized experiment control, data acquisition and analysis were performed by LabView based software developed in our laboratory.
RESULTS AND DISCUSSION
Chemical reaction of lidocaine with singlet oxygen.
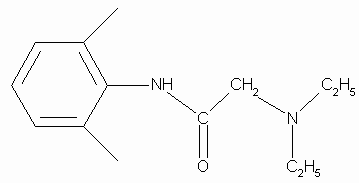
Rate constants for the chemical reaction between lidocaine and O2(1D g) were determined in methanol, acetonitrile and N,N-dimethylformamide. Lidocaine consumption was followed during the reaction. Rate constants for the chemical reaction, kRLID, are (1.05 ± 0.061) x 105 M-1 s-1, (1.42 ± 0.073) x 105 M-1 s-1 and (0.61 ± 0.046) x 105 M-1 s-1 in acetonitrile, methanol and N,N-dimethylformamide, respectively.By using the Mair method (4) for hydroperoxide determination, a concentration equivalent to 0.0153 M of hydroperoxide was found when 0.03 M lidocaine in acetonitrile was irradiated for 12 h in the presence of Rose Bengal. The amount of hydroperoxide produced agrees with the consumption of lidocaine. Although we cannot isolate reaction products in quantities adequate for spectroscopic characterization, a rough idea of the product distribution was obtained by GC-MS analysis of the main lidocaine derivatives produced in the photooxidations. When 0.03 M lidocaine was irradiated for 12 h in the presence of Rose Bengal the results shown in Fig. 2 a) are obtained with the mass spectrometer in the positive chemical ionization (CI+) mode. Only four peaks appear in the chromatogram, the main one, with a retention time of 15.59 min, is that of unreacted lidocaine. Fig. 2 b) shows that the mass spectrum is that of lidocaine. The CI+ and EI (not included) mass spectra corresponding to peaks at retention times of 14.57, 13.22 and 7.77 min, indicate that 2-(ethylvinylamino)-N-(2,6-dimethylphenyl)-acetamide, 2-(1-azapropily-den)-N-(2,6-dimethyl-phenyl)-acetamide and 2,6-dimethylaniline are the probable main products of photooxidation of lidocaine. Figs. 2 c), 2 d) and 2 e), show the CI+ mass spectra and corresponding structures.
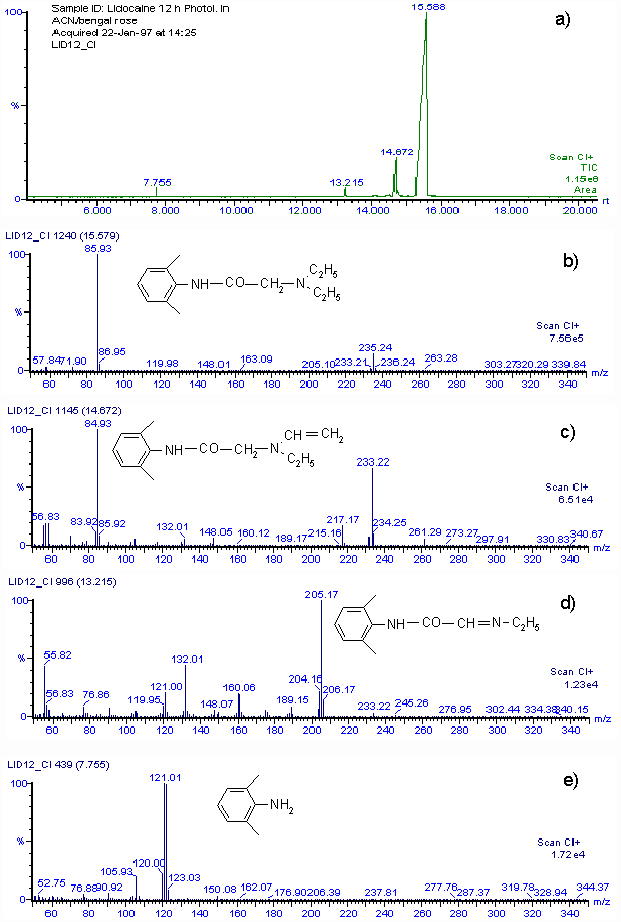
Figure 2.a) GC-MS chromatogram of 30 mM lidocaine in acetonitrile after 12 h of irradiation in the presence of Rose Bengal. b) CI+ mass spectrum of compound with retention time 15.58 m. c) CI+ mass spectrum of compound with retention time 14.67 m. d) CI+ mass spectrum of compound with retention time 13.22 m. e) CI+ mass spectrum of compound with retention time 7.76 m.
Physical quenching of singlet oxygen by lidocaine. In most of the studied solvents, total rate constants, kTLID, for the reaction of lidocaine with O2(1D g), were determined by using time-resolved phosphorescence. Figure 3, shows a typical Stern-Volmer plot for quenching of singlet oxygen by lidocaine. Values of kTLID, in different solvents, were obtained from slopes of these plots. The insert shows the luminescence decay of singlet oxygen at 1270 nm in acetone as solvent and Rose Bengal as sensitizer. Decays obtained over several concentrations of lidocaine are included in this figure. The lifetime of O2(1D g) was obtained from single exponential decays in the absence or presence of variable concentrations of lidocaine.
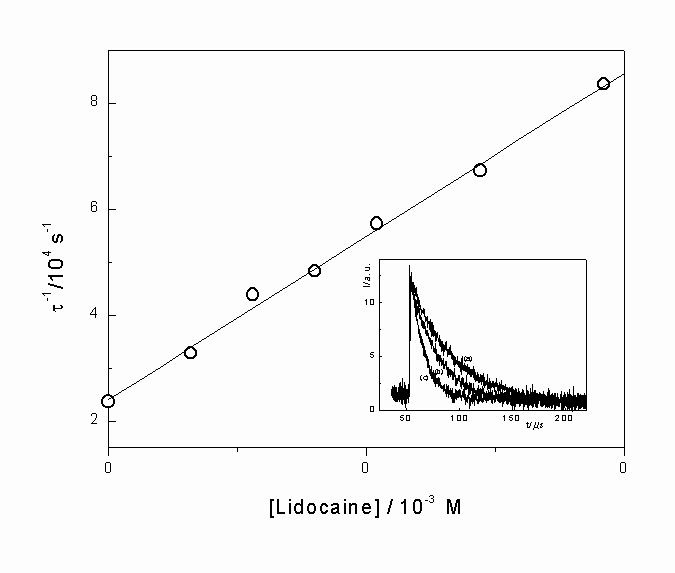
Figure 2. Stern-Volmer plot for deactivation of singlet oxygen by lidocaine in acetone as the solvent. Insert (a) Singlet oxygen phosphorescence decay at 1270 nm, following dye laser excitation at 414 nm, with Rose Bengal as sensitizer in acetone. (b) and (c) as a), but with 1.6 mM and 9.6 mM of lidocaine, respectively.
Table 1
|
Solvent |
kTLID/106 M-1 s-1 |
kTTEA/106 M-1 s-1 |
|
|
1 |
n-Heptane |
1.10 ± 0.08c |
68.8 ± 3.4 |
|
2 |
n-Hexane |
1.24 ± 0.05ª |
66.2 ± 2.6 |
|
1.29 ± 0.06b |
|||
|
3 |
Diethylether |
3.30 ± 0.16b |
91.5 ± 3.2 |
|
4 |
Dioxane |
2.53 ± 0.13b |
275.2 ± 9.1 |
|
5 |
Ethyl Acetate |
5.26 ± 0.17b |
190.4 ± 7.6 |
|
6 |
Tetrahydrofurane |
7.02 ± 0.32b |
221.1 ± 6.6 |
|
7 |
Benzene |
2.00 ± 0.11c |
198.5 ± 7.1 |
|
2.69 ± 0.09a |
|||
|
8 |
Tributylphosphate |
39.4 ± 0.16b |
71.4 ± 3.5 |
|
9 |
Anisole |
3.67 ± 0.19b |
314.8 ± 9.4 |
|
10 |
Propylene Carbonate |
8.15 ± 0.36b |
258.1 ± 8.9 |
|
11 |
N,N-dimethylformamide |
31.70 ± 1.27b |
347.6 ± 10 |
|
12 |
N,N-dimethylacetamide |
45.8 ± 2.40a |
422.2 ± 12 |
|
13 |
Benzonitrile |
4.28 ± 0.21b |
254.2 ± 9.1 |
|
14 |
Acetone |
6.14 ± 0.28b |
216.7 ± 7.6 |
|
15 |
Methylene chloride |
1.42 ± 0.06b |
128.4 ± 5.1 |
|
16 |
Acetonitrile |
2.36 ± 0.09d |
165.1 ± 6.6 |
|
3.17 ± 0.15a |
|||
|
4.10 ± 0.19b |
|||
|
17 |
Chloroform |
0.98 ± 0.05a |
45.3 ± 2.3 |
|
0.90 ± 0.04b |
|||
|
18 |
Benzylic alcohol |
2.48 ± 0.11b |
15.2 ± 0.8 |
|
19 |
Formamide |
2.81 ± 0.12a |
21.6 ± 1.1 |
|
20 |
i-Propanol |
4.30 ± 0.22b |
27.8 ± 1.4 |
|
21 |
n-Octanol |
3.40 ± 0.16b |
30.4 ± 1.6 |
|
22 |
n-Hexanol |
- |
22.2 ± 1.0 |
|
23 |
i-Pentanol |
3.04 ± 0.13b |
19.7 ± 1.2 |
|
24 |
n-Pentanol |
3.52 ± 0.16b |
26.2 ± 1.3 |
|
25 |
n-Butanol |
3.54 ± 0.14b |
22.3 ± 1.1 |
|
26 |
n-Propanol |
2.96 ± 0.12a |
14.8 ± 0.7 |
|
27 |
Ethanol |
2.72 ± 0.13d |
23.6 ± 1.4 |
|
2.93 ± 0.15a |
|||
|
28 |
Methanol |
2.08 ± 0.09a |
12.7 ± 0.5 |
|
29 |
Trifluoroethanol |
0,20 ± 0.09a |
- |
a
sensitizer : Phenazine or TPP, Nd-YAG Laserb
sensitizer : TPP or Rose Bengal, Dye Laserc
sensitizer : Rubrene, Steady State Methodd
sensitizer : Rose Bengal, Steady State MethodA comparison of kRLID and kTLID values shows that kTLID is greater than kRLID by between one and two orders of magnitude in the same solvent, indicating that the main path for interaction of lidocaine with singlet oxygen corresponds to physical quenching of excited oxygen by the anaesthetic, thus the rate constant for this process, kQ, approximately equals kTLID.
Quenching of singlet oxygen by aliphatic amines is well studied (5,6), and is explained in terms of reversible formation of an exciplex, via charge-transfer interactions, due to electrophilic attack of excited oxygen on the amino group. The exciplex yields products by chemical reaction or undergoes intersystem crossing to regenerate amine and triplet oxygen.
This behavior, expected for lidocaine, fits our steady state and time resolved experiments. Photooxidation product distribution, hydroperoxide detection, dependence of kTLID on pH for reaction in aqueous solution and the increase of lidocaine reactivity towards singlet oxygen in more polar aprotic media, indicate that reaction between singlet oxygen and lidocaine occurs via formation of a charge transfer exciplex followed by physical quenching or chemical reaction. Scheme 1 shows a mechanism compatible with these observations.
Scheme 1
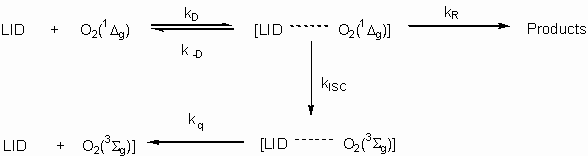
However, although these results are consistent with a common mechanism for reactions of lidocaine or aliphatic amines with singlet oxygen, several differences require additional explanations. Mainly, values of kTLID are between one and two orders of magnitude lower than those previously reported for typical tertiary amines (7). This lower reactivity of lidocaine relative to tertiary amines is not easily understood in terms of steric effects and we note that dependence of kTLID on solvent has a different pattern from that for amines (7). For instance, kT values in acetonitrile are (2.36 ± 0.09) x106 M-1 s-1 and 33.3 x 107 M-1 s-1 (21) while in methanol, values of kT are (2.08 ± 0.09) x 106 M-1 s-1 and 1.3 x 107 M-1 s-1 for lidocaine and triethylamine, respectively. Tertiary aliphatic amines dramatically decrease their reactivities towards singlet oxygen when the media changes from aprotic polar solvents, such as acetonitrile or methylene chloride, to protic polar solvents such as aliphatic alcohols. In contrast to this well-established behavior, lidocaine reactivity in aprotic polar solvents is very close to that in aliphatic alcohols.
In order to understand the solvent effect on kTLID and explain the lower reactivity of the drug we measured the rate constant for reaction between triethylamine (a typical tertiary amine) and singlet oxygen, kTTEA, in the solvents employed to determine kTLID. These results are included in Table 1.
LSER and TLSER analysis of solvent effect on kTLID and kTTEA. With the aim of rationalizing differences in lidocaine reactivity relative to that typical of aliphatic amines and solvent effects on kTLID, we analyzed the kT dependence on microscopic solvent characteristics by using the semiempirical solvatochromic equation (LSER) of Taft, Kamlet et al. (Eqn. 1) (8).
Log k = log ko + s (p * + d d ) + a a + b b + h r 2H (1)
Also, we analyze the dependence of kTLID on the solvent by using a theoretical set of correlation parameters determined solely from computational methods. The theoretical linear solvation relationship (TLSER) descriptors have been developed to give optimal correlation with the LSER descriptors. The generalized TLSER equation proposed by Famini et al. (9,10), (Eqn. 2) can be used to analyze chemical reactivity.
Log k = log ko + a r 2H + b p 1 + c e b + d q- + e e a + f q+ (2)
The correlation equations obtained for the dependence of kTLID and kTTEA on the solvent parameters are included in Table 2. The results show that not all the descriptors are significant. Descriptor coefficients accepted in the correlation equation were those that have a significance level ³ 0.95.
Table 2
. LSER and TLSER correlation equations for the reactions of singlet oxygen with lidocaine and triethylamine.|
Log k = Log ko + a p * + b a + c b |
||||
|
TEA |
Log ko |
a |
b |
c |
|
Coeff. |
7.924 |
0.321 |
-1.305 |
0.362 |
|
± |
0.111 |
0.151 |
0.134 |
0.184 |
|
t-stat. |
71.126 |
2.121 |
-9.705 |
1.967 |
|
P(2-tail) |
< 0.0001 |
0.0444 |
< 0.0001 |
0.0609 |
|
VIF |
1.068 |
1.721 |
1.869 |
|
|
N = 28 R = 0.927 SD = 0.200 F = 48.995 |
||||
|
LIDOCAINE |
Log ko |
a |
b |
c |
|
Coeff. |
5.946 |
0.425 |
-0.813 |
1.318 |
|
± |
0.096 |
0.128 |
0.081 |
0.121 |
|
t-stat. |
62.106 |
3.318 |
-10.074 |
10.872 |
|
P(2-tail) |
< 0.0001 |
0.0029 |
< 0.0001 |
< 0.0001 |
|
VIF |
1.014 |
1.144 |
1.159 |
|
|
N = 28 R = 0.941 SD = 0.173 F = 61.306 |
||||
|
Log k = Log ko + d p 1 + e q+ + f q- |
||||
|
TEA |
Log ko |
d |
e |
f |
|
Coeff. |
6.465 |
15.031 |
-6.047 |
1.254 |
|
± |
0.487 |
4.109 |
0.647 |
0.372 |
|
t-stat. |
13.278 |
3.658 |
-9.344 |
3.374 |
|
P(2-tail) |
< 0.0001 |
0.0015 |
< 0.0001 |
0.0029 |
|
VIF |
1.109 |
1.160 |
1.193 |
|
|
N = 25 R = 0.919 SD = 0.215 F = 37.967 |
||||
|
LIDOCAINE |
Log ko |
d |
e |
f |
|
Coeff. |
4.734 |
12.022 |
-2.655 |
2.717 |
|
± |
0.633 |
5.309 |
0.901 |
0.514 |
|
t-stat. |
7.476 |
2.264 |
-2.947 |
5.290 |
|
P(2-tail) |
< 0.0001 |
0.0343 |
0.0077 |
< 0.0001 |
|
VIF |
1.143 |
1.193 |
1.164 |
|
|
N = 25 R = 0.786 SD = 0.299 F = 11.323 |
||||
From the correlation equations listed in Table 2 for the LSER approach applied to the reactions of singlet oxygen with lidocaine and triethylamine we see that: i) Coefficients for the p * parameter obtained for both lidocaine and triethylamine are very similar. This result supports the proposed formation of an exciplex with a considerable charge transfer character. ii) Coefficients associated with the a parameter are negatives for both lidocaine and TEA, with the a coefficient being statistically more significant in the equation for TEA. iii) The b coefficients are positive for both, lidocaine and TEA and correlations equations indicate that HBA solvents contribute to exciplex stabilization. In the LSER correlation for lidocaine, the b parameter coefficient is the most important and larger than the corresponding parameter in the TEA correlation. In addition the a parameter coefficient in the lidocaine LSER equation is lower than in the triethylamine LSER equation.
The TLSER correlation gives similar results to those obtained with LSER. In Table 2 one can see that for both, lidocaine and TEA, coefficients corresponding to the solvent polarizability p 1 parameter are very similar, and have the largest statistical significance in the correlations. Also, the TLSER treatment shows that HBD solvents inhibits the reaction of singlet oxygen with both, lidocaine and TEA, however, the coefficient for q+ in the TEA equation is larger more than a factor two than that in the lidocaine equation. Concerning this point we note that the TLSER analysis indicates that influences of HBD solvents are mainly electrostatic, because only q+ is included in the correlation equation although, for the solvent set studied, a shows a very good correlation with the theoretical parameters e A and q+ (a = 0.820 - 5.803 e A + 4.723 q+; R = 0.975; F = 211.94). Additionally, the TLSER correlation shows that HBA solvents increase the reaction rate although the relative importance of the coefficient associated with the q- parameter is lower than that for b in the LSER analysis.
The meaningful differences found for solvent effects on kTLID and kTTEA, can be understood if solvents have specific effects on lidocaine and TEA reactions. The decrease of kTTEA in HBD solvents is explained in terms of hydrogen bonding interactions between solvent and the amino nitrogen, which sterically hinders O2(1D g) access to this nitrogen inhibiting exciplex formation. The effect of HBD solvents on kTLID is more complex. Lidocaine has an electron withdrawing amido group near to the reaction site, which decreases the electronic density of the reactive nitrogen atom as predicted by simple semiempirical quantum mechanical calculations. Determinations of charge densities by using MOPAC 97 with AM1 Hamiltonian show that electron density on the aminic nitrogen of lidocaine is lower than that on the nitrogen of TEA, which explains the lower reactivity of lidocaine towards singlet oxygen as compared with TEA. However, the expected decrease in lidocaine reactivity with a change from aprotic to protic solvents was not observed. This behavior may be rationalized if inductive electron withdrawal by the amido group is modified by solvent-lidocaine interactions or if hydrogen bonding between the nitrogen atom of the amino group and the solvent is weaker than in a typical tertiary amine such as triethylamine. 13C-NMR spectra of lidocaine in methanol, methanol-D4, and carbon tetrachloride show that in methanol and in methanol-D4 the signal corresponding to the carboxylic carbon of the amido group is at 172.5 and 172 ppm, respectively, whereas in carbon tetrachloride it is at 167.5 ppm. These results allow us to disregard changes in electron withdrawal by the amido group as being responsible for the unexpectedly high reactivity of lidocaine in aliphatic alcohols. Furthermore, we expect that the strength of hydrogen bonding interactions will be decreased due to the low electron density on the amino nitrogen of lidocaine as compared with that in TEA. Additionally, a cooperative participation of the amido oxygen to hydrogen bonding that weakens the HBD solvent-aminic nitrogen interaction may be considered. Both effects may explain the lower dependence of kT on the a parameter for reaction between lidocaine and singlet oxygen. Rate effects of HBA solvents on the reaction of singlet oxygen with lidocaine and TEA can be understood in terms of stabilization of the exciplex by solvents with the highest b or q- values. Also, we can conclude that the interaction between HBA solvents and the exciplex is mainly electrostatic, because the TLSER correlation shows that the reaction rate depends largely on the q- parameter and is almost independent of e B. We note that in the solvents employed in this study, there is a reasonable correlation for the dependence of b with e B and q- (b = 0.494 - 3.555 e B + 1.826 q-; R = 0.782; F = 17.273 ). The larger statistical significance of b (or q-) for lidocaine as compared with TEA, can be explained if intramolecular interactions between the amido NH and the tertiary amino group are considered. Thus, HBA solvents compete for the anilide H-bond donor group of lidocaine, diminishing the tendency for intramolecular hydrogen bonding or intramolecular electrostatic stabilization with the tertiary amino centre. As result, HBA solvents free the reactive centre for interaction with singlet oxygen, thus increase the quenching rate.
In conclusion, our data show that lidocaine is a moderate quencher of singlet oxygen. This result, in addition to its ability to trap free radicals explains, at least partially, the protective role of this anaesthetic in biological systems. Also, our data show that the lower reactivity of lidocaine towards singlet oxygen relative to that of triethylamine, is a consequence of electronic factors and that steric effects are not significant. Additionally, the solvent dependence of singlet oxygen quenching rate by lidocaine, as distinct from that for typical tertiary amines, is due to the presence of the amido group in lidocaine. LSER and TLSER analyses are valuable tools in understanding these results. Finally, it is important to recognize that hydroperoxide products that could arise from the reaction between lidocaine and singlet oxygen can initiate oxidations in cellular media at physiological pH.
REFERENCES