| Papers and Posters | Site Home Page |
Photolysis of pyrene by UV radiation
Pavel Kubát, Svatopluk Civiš J. Heyrovský Institute of Physical
Chemistry, Academy of Sciences of the Czech Republic |
Alexander Muck, Jiří Barek, Jiří Zima Department
of Analytical Chemistry, Faculty of Science, Charles University |
Contact: kubat @jh-inst.cas.cz
![]()
| Introduction | Exprimental | Rate of photolysis | Mechanism of photolysis | Conclusions | References |
![]()
Abstract
| The photolysis of pyrene (Py) in the presence of acetic acid (AA) occurs through a radical mechanism and is faster than in organic solvents (methanol). PyH·(absorption maximum in the UV/VIS spectra lmax = 395 nm in AA, lifetime t = ~2 m s in oxygen-saturated AA) was identified as an intermediate. Absorption of a photon by ground state of Py leads to the formation of excited states (1Py* and 3Py), which deexcite to the ground state without chemical changes. Singlet oxygen 1O2 formed by energy transfer from 1Py* and 3Py to dissolved oxygen does not react with Py. PyH-· and PyH+· were not found following excitation under the given conditions. The rate constant of photolysis can be considerably increased by addition of water and hydrogen peroxide. The accelerating effect of hydrogen peroxide consists in photolytic formation of reactive hydroxyl radicals, which attack the fused benzene rings. |
![]()
Polycyclic aromatic hydrocarbons (PAH) and their derivatives constitute one of the largest groups of chemical carcinogens and mutagens [1]. These are primarily anthropogenic pollutants that enter nature from industrial exhalations. The chief sources of PAH consist of household heating by coal, coal-fired power stations, automobile traffic, cigarette smoke and emissions from waste incineration plants. PAH with polar substituents were found in natural waters; the less polar compounds are important contaminants in sediments, soils and sludges [2]. The enhanced phototoxicity of PAH consists in the formation of reactive oxygen species via energy transfer from excited PAH states, which produce toxicity through oxidative stress [3]. Consequently, there is an increasing demand for the determination of trace concentrations of these substances and removal from the natural environment. Several chemical methods have been proposed and tested [4]. These methods are based on chemical oxidation, e.g. using potassium permanganate in acidic and alkaline media, using concentrated sulfuric acid or by direct ozonation or catalytic oxidation methods. Although UV radiation in small amounts, e.g. in sunlight, can lead to increased toxicity of PAH and the formation of more toxic species, irradiation with artificial sources with higher power in the presence of radicals could lead to decomposition of the fused benzene rings. Methods based on photolytic degradation could be both effective and easy. In addition, information on the photolytic destruction of environmental carcinogens obtained during the development of these methods can be useful in elucidation of the fate of these substances in the environment. This poster is concerned with the effect of the surrounding medium (solvent) on the rate and mechanism of the photolysis of pyrene (Py) as a model compound of the PAH group. Attention is paid to the initial phase of the process (to several ms following absorption of a photon), when the very stable aromatic skeleton of Py is broken. The mechanisms of photolysis are studied primarily by methods of time-resolution spectroscopy, which permit monitoring of the individual intermediates formed following excitation of Py. |
![]()
Experimental
|
Figure 1 Experimental set up for (time-resolved) spectroscopy 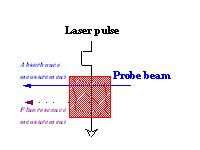 click to enlarge |
Lambda Physik LPX 205 excimer laser (l =308 nm, pulse
length 28 ns) for pulse irradiation 900 W xenon discharge lamp with a monochromator for the continuous irradiation (irradiation wavelength 324 ± 18 nm, power 30 mW, Applied Photophysics, UK) Laser kinetic spectrometer (Aplied Photophysics) [5] for measurement of time-resolved fluorescence and absorption spectra Perkin Elmer Lambda 10 UV/VIS spectrometer |
![]()
Rate of photolysis of Py in different enviroment
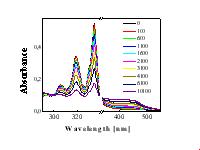
Rate constant of photolysis of Py, kpyr, is strongly influenced their environment. kpyr is four times higher in AA than in methanol and increase significantly in the presence of water and hydrogen peroxide, which is source of reactive ·OH radicals (Table 1). Continuous or pulse irradiation yielded products with identical UV/VIS spectra and the same order of relative kpyr values. No induction period was observed. The products of photolysis are different in these two cases: in methanol (organic solvent), primarily products absorbing in the UV region are formed (not shown), while also products, apparently of the quinone type, absorbing in the 400 - 500 nm region are formed in the presence of AA (Figure 2). |
Figure 2 UV/VIS spectra of 13 mM Py in AA irradiated by XeCl laser (energy 9.5 mJ/pulse) after 0, 100, 600, 1100, 1600, 2000, 3000, 4000, 6000 pulses
|
Table 1 Initial rate constant kpyr of the photolysis of a 50 mM Py solution by continuous radiation (lirr=324 ± 18 nm) in various enviroments
*) estimated error ± 15 % |
| Absorption spectra of intermediates formed by photolysis and radiolysis of pyrene are not greatly affected by the solvent and are well described in the literature. The following absorption and emission bands were used for detection: |
| Ground state of Py | Absorption band at 334 nm |
| Excited singlet states 1Py* | Emission band at 390 nm (quenched by oxygen), lifetime less than 30 ns in oxygen saturated AA |
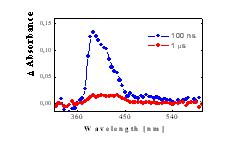
| Triplet states 3Py | Absorption band at 415 nm [6] (quenched by oxygen, Figure 3), lifetime of 220 ns in oxygen saturated AA |
| Radical PyH· | Absorption band at 390 nm [7] (partially overlaied by
triplet states, Figure 3) lifetime of 2.1 ms in oxygen saturated AA |
| Excimer (Py...Py)* | Broad emission band at 460 nm, negligible at concentration less than 10 mM |
| Radical cation PyH+· | Absorption band at 465 nm (not found) |
| Radical anion PyH-· | Absorption band at 460 nm (not found) |
| Mechanismus of the formation of 1Py*, 3Py and 1O2
after absorption of a photon in organic solvents is extensively described in literature [8,9] (Eq. 1-3).
1Py*
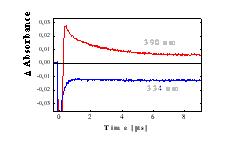
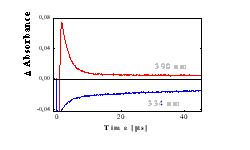
3Py The quantum yield of 1O2 ( FD) for Py in methanol is greater than 0.6 [9]. Thus, if O2 is formed only by energy transfer from 1Py* and 3Py(FD< 1), the increase of kpyr in presence of AA and H2Oby two orders of magnitude (Tab. 1) cannot be explained. For this reason, Py in the ground state, 1Py* and 3Py do not significantly react with O2 in either the ground state or in the excited 1Dg state. The single-exponential decomposition kinetics (Fig. 4b) indicates that PyH· is no longer formed at time intervals longer than 100 ns after the laser pulse. At shorter times, the PyH· band is overlapped by the 3Py band and the intense fluorescence of 1Py*, and thus we can only speculate on the mechanism of the formation of PyH· . Charge-transfer complexes of Py and the anhydride of organic acids, whose excitation and subsequent decomposition lead to the formation of ion pairs Py- and An+, are described in the literature [10]. Similar processes can also be expected in the case of excitation of Py-AA complexes: (a) formation of the excited Franck-Condon state of the charge-transfer complexes, (b) charge redistribution within the complex and (c) subsequent dissociation with formation of PyH· (Eq. 4) The different reactivities of 3Py nad PyH· can be demonstrated in oxygen-saturated AA (Fig. 4), where lifetime of 1Py* and 3Py is less than 250 ns. At times longer than 1 m s following the laser pulse, the concentration of the ground state of Py (formed by deexcitation of 1Py* and 3Py) no longer increases (see points A in Fig. 4a). The changes in the absorbance DA (Fig. 4a) is constant and is caused by formation of products absorbing at higher wavelengths. Thus, PyH· , which is still present on the microsecond time scale, decomposes further to form products or reacts with AA by a radical reaction, but not with formation of the ground state of Py. At times longer than 8 ms, D A (Fig. 4b)no longer changes at a wavelength of 390 nm. The increase in absorbance D A compared to the value prior to the laser pulse is caused by the formation of the final products (Fig. 2c). The decomposition of Py in oxygen-saturated AA is thus terminated within a time period of 10 ms after photon absorption. In He-saturated and partially in air-saturated solution are PyH· overlapped by band of 3Py , but the photolysis occur by the same mechanism (Fig. 5). |
|
Figure 3 Absorption spectra of PyH and 3Py in air-saturated solution*) , 100 ns (blue line) and 1 ms (red line) after excitation pulse |
Figure 4 Changes in the absorbance at 390 nm (PyH· , red line) and of ground state of Py (blue line) vs time in oxygen-saturated solution*) |
Figure 5 Changes in the absorbance at 390 nm (PyH·, red line) and of ground state of Py (blue line) vs time in He purged solution*) |
___________________________
*) measured in acetic acid : water 1:1
![]()
Conclusions
|
![]()
Acknowledgements
The financial support of the Grant Agency of the Czech Republic (Grant No. 203/98/1187) is gratefully acknowledged
![]()
| Number of
visitors: |
Created by NETSCAPE 4.6, last update June 17, 1999 |


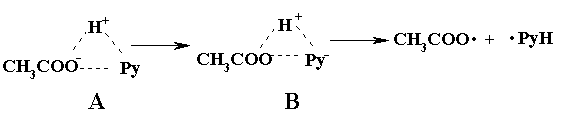 (4)
(4)