Photophysics and Photochemistry of Bifluorophore Systems
N. Vasil'eva and I. Sokolova
Department of Photonics of Molecules
Siberian Physical-Technical Institute by Tomsk State University
Revolution sq., 1
634050 Tomsk
Russia
E-mail: root@eccspti.tomsk.su
Fax: (8-382-2) 23-30-34
SUMMARY
Theoretical and experimental studies of photophysical and photochemical processes for nonconjugate bichromophores, i.e. molecules of two different monofluorophores coupled by (CH2)n-bridge, are present. Our investigations have shown that bichromophores are more complex molecular systems, than a mere sum two nonconjugate fluorophores. Bichromophore is a unified system - "supermolecule" with the unified system of molecular orbitals largely belonging to separate fragments, but on the other hand, unified molecular orbitals. It was obtained after optical excitation in the donor absorption band there develop fast internal conversion processes with the result that the molecule is found in the S1*-state localized on the acceptor fragment. Thus the energy transfer from the donor fragment to the acceptor that may be interpreted as the internal conversion process.
INTRODUCTION
The coumarin derivatives are at one of the first places among the most effective laser dyes in the blue-green spectral region that are know now, but the relation of their structure to spectral parameters of their luminescence and photochemical properties have been investigated insufficiently. We consider results of experimental and theoretical research of the bichromophore systems based on stilbene and aminocoumarins. The theoretical and experimental study of dynamics of photophysical processes, photoreactivity, spectral and lasing properties in polyatomic organic molecules is the principal direction of our research.
In this study we deal with nonconjugate bichromophores, i.e. molecules composed of two different monofluorophore molecules coupled by (CH2)n-bridge. A bichromophore consists of a donor molecule with a higher energy in the first excited singlet state (S1D) than the energy of an acceptor molecule in the lower S1A state. The bichromophores are of great interest for quantum electronics, for developing systems of optical filtration and conversion of lamp and laser radiation, for constructing scintillation detectors, etc. [1, 2].
Trans-stilbene and 4-methylumbelliferone have been used as donor fragments, and amino-coumarins of different structure have been used as acceptor fragments (Fig. 1). We consider results of experimental and theoretical research into three bichromophore molecules: trans-stilbene - CH2 - coumarin 120 (linear bichromophore), 4-methylumbelliferone - CH2 - UC17 and 4 - (3-fluoro) - methylumbelliferone - CH2- UC17 (nonlinear bichromophores) in detail (Fig. 1).
THEORETICAL AND EXPERIMENTAL METHODS
The theoretical basis of our approach are concepts and methods of quantum chemistry and a theory of nonradiative transitions in polyatomic organic molecules. To develop the quantum chemical algorithms and programs the semiempirical method with intermediate neglect of differential overlapping (INDO) is used which involves particular spectroscopic parametrization [3]. This approach allows the spectra of energy states to be calculated with an error not larger than 5 - 10 per cent and provides the basis for dynamics of ultrafast processes occurring in this kind of organic compounds to be studied.
The calculations have been performed to determine the form of molecular orbitals (MO), the electron density distribution in the ground and excited states and the nature of the electronic excited states of test molecules. The nature of the electronic excited states has been analyzed on the basis of the calculated expansion coefficients of MO in terms of the atomic 2s, 2px, 2py and 2pz-orbitals and wave functions of electronic-excited states to fit the Slater configurations. Wave functions of the excited electron states are represented in the form of the linear expansion in terms of single excited configurations | i-> k>:
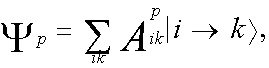 . . (1)
. . (1)
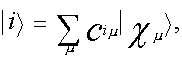 . . (2)
. . (2)
where | i > is molecular orbital (MO), | Xµ > is atomic orbital (AO).
The calculations have been performed to determine the form of molecular orbitals (MO), the electron density distribution in the ground and excited states and the nature of the electronic excited states of test molecules. The intermolecular donor-acceptor interactions (in particular, protoning processes) was studied using molecular electrostatic potential (MEP) method [4]. Our use of the MEP allowed us to determine the most probable path of a proton and, hence, to receive information about the gas-phase basicity of the molecules. The rate constant of internal conversion kic involving the electron states g and f can be estimated using the following formula:
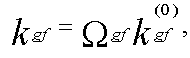 . . (3)
. . (3)
where kgf(0) is the rate of the g->f internal conversion in the single configuration approximation, omegagf is the factor describing overlapping of the wave functions of the electronic states g and f [5]. A computer program is developed for calculating the matrix elements of the spin-orbital interaction operator to estimate the rate of singlet-triplet conversion (kST) [6].
The emission properties have been investigated under excimer XeCl*-laser excitation [7]. The laser pulse energy is up to 20-50 mJ, the pulse duration is 10 ns and the pump wavelength is lambda = 308 nm. The absorption and fluorescence excitation spectra of the ethanol solutions with concentration of 10-6 M have been studied using spectrophotometer "Specord M40". Recording of luminescence spectra and their integration for determination of quantum yield were carried out by means of spectrofluorimeter "Hitachi - 750. Coumarin 1 in ethanol was used as a standard for quantum yield determination (Ø).
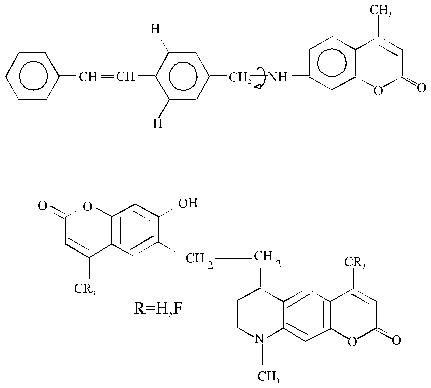
Fig. 1 - Structure formulae of the test bichromophores. I - TS - CH2 - C120; II - 4MU - CH2 - UC17; III - 4FMU - CH2 - UC17.
EXPERIMENT
The absorption and excitation fluorescence spectra of the ethanol solutions (it is =< 10-6 M) and fluorescence quantum yield under excitation in the acceptor region were studied with spectrophotometer "Specord M40". The absorption spectra of TS, C120 and bifluorophore TS-CH2-C120 in ethanol are shown in Fig.2, panel a. The absorbency of C120 and trans-stilbene fragments increases by 10 and 12 per cent respectively, as compared to the absorbency of corresponding incoherent molecules. The TS extinction coefficient of stilbene (e) is as high as ca. 30 103 M cm-1. Furthermore, the absorption maxima of the stilbene and coumarin fragments of TS-CH2-C120 occur at longer wavelength (lambda = 320 nm and 345 nm) than maxima of single molecules TS and C120 (lambda = 312 nm and 340 nm). In addition it is evident from Fig. 2 that the absorption spectrum of the TS-CH2-C120 bichromophore as a whole is not an additive sum of the spectra of its constituent fragments (TS and C120). The fluorescence yield of stilbene is very low (Ø = 0.02) [8] by virtue of high trans-cis photoisomerization rate. The quantum yield for trans-cis photoisomerization is 0.5 in the absence of bimolecular quenching of TS* [9]. The bichromophore fluorescence is assigned to C120 emission (lambda = 440 nm). From this point of view the energy transfer efficiency should be fairly high. Really, the fluorescence yield of TS-CH2-C120 is the same that of C 120.
Figure 2 (panel b and c) shows that the absorption spectrum of the nonlinear bifluorophores as a whole is not an additive sum of the spectra of its constituent fragments. From Table I it is seen that the fluorescence resulting from nonlinear bichromophores depends strongly on the excitation wavelength. When the excitation wavelength is in the donor absorption region the fluorescence yield of these bichromophores is less than that under excitation in the acceptor region. The fluorescence of both linear and nonlinear bichromophores is assigned to an acceptor emission, and the fluorescence of a donor is absent in the case.
DISCUSSION
Quantum chemical calculations by the INDO method have been done to determine the schemes of photophysical processes in the bichromophore molecules. In this study we present the absorption spectra calculated for TS, C120, 4MU, 4FMU, UC 17 and bichromophores created on its basis. Figures 3-5 give the calculated energies of the singlet and triplet levels, oscillator strengths of the lower singlet excited states (f) , the rate of the internal conversion kic and the rate of the singlet-triplet conversion of electron transitions. Analysis of the electronic excited states shows that the internal conversion process is the basic channel of the excitation energy degradation (kic = 1012 s-1) for the test molecules.
Table 1.Spectral properties of the test bichromophores (j - fluorescence yield).
Molecules |
j A*) |
|
|
|
4MU- CH2- UC17 |
|
1.0 |
0.8 |
|
4FMU- CH2- UC17 |
|
0.8 |
0.6 |
|
TS - CH2 - C120 |
|
1.0 |
1.0 |
Note: *) - fluorescence yield of acceptor; **) - relation of fluorescence yield bichromophore to acceptor at excitation in the acceptor region; ***) - relation of fluorescence yield bichromophore to acceptor at excitation in the donor region .
Previously [11, 12] we have shown that MO of bichromophore TS-CH2-C120 by their nature are predominantly either the donor or acceptor MO. It should be noted that , when the bichromophore is formed, the energies of the upper occupied MO increase for coumarin fragment and decrease for stilbene fragment (0.2 - 0.3 eV). The ionization potential of the C120 molecule is higher than that of the TS molecule, i.e. coumarin , being an energy acceptor (A), may be as an electron donor (D) with respect to stilbene.
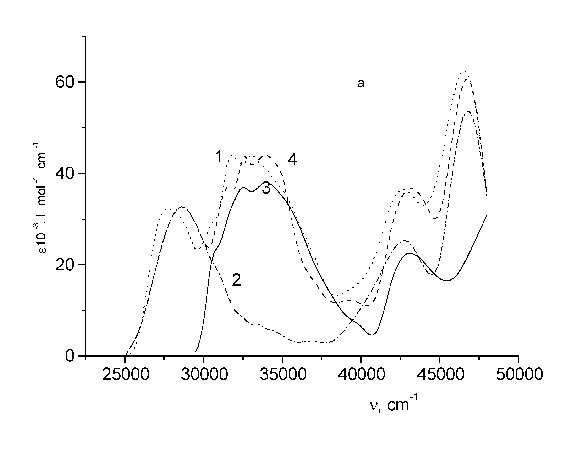
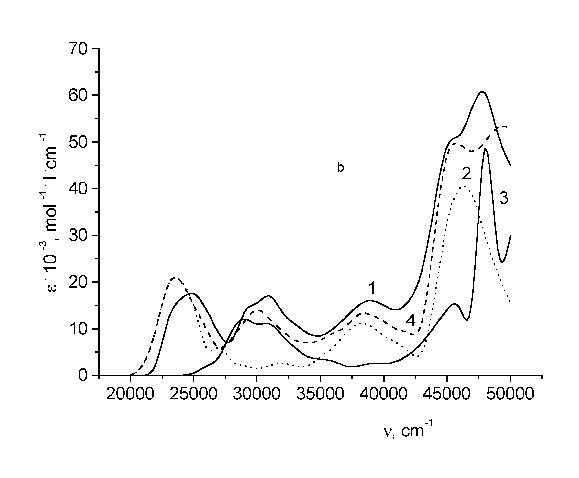
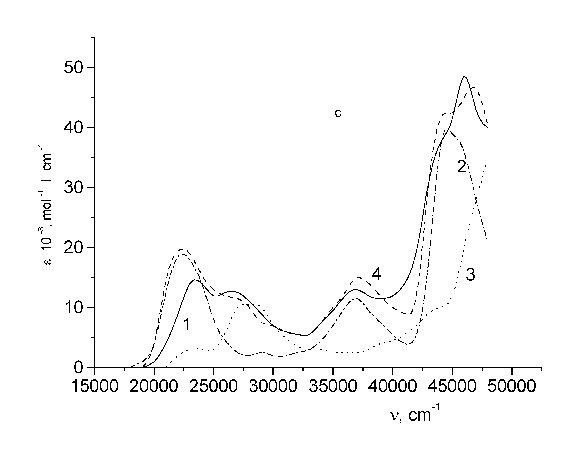
Fig. 2 - Absorption spectra of: (a) TS - CH2 - C120 (I); (b) 4MU - CH2 - UC17 (II); (c) 4FMU - CH2 - UC17 (III). In all panels, curves are: 1 - spectrum of bichromophore; 2 - acceptor; 3 - donor; 4 - equimolar solution.
Fluorination of the methyl group of the C120 molecule decreases the energy of the occupied MO, even though fluorine atoms do not contribute to the structure of the upper occupied MO of pi-type. The fluorine atoms produce strong induction effect both on the pi-type and, to a greater extent, on the sigma-type orbitals.
A marked difference between the MO energies has been observed for high energy vacant sigma*-orbitals (1,5 eV) in TS-CH2 -FC120. The appearance of two fluorine atoms in the stilbene fragment significantly affected positions of the vacant pi* -MO and sigma* -MO [2, 12].
As shown in [2, 12] the introduction of the trifluoromethyl group in the acceptor brings about a bathochromic shift of the first band absorption band and a redistribution of the oscillator strength between the two lower transitions make up the first absorption band. Similar changes occur under fluorination of the methyl group of the C120 molecule. Likewise, substitution of two hydrogen atoms for two fluorine atoms also leads to bathochromic shifts of singlet states localized on the energy donor in FTS-CH2 -C120 and the very number of mixed states increases as well.
Recognizing the mixed character of the electronic states of the test bichromophores and treating them as a single molecular system we have assumed the following scheme for photophysical processes in the bichromophore molecules. After optical excitation of the strong donor absorption band there develop fast internal conversion processes with the result that the molecule is found in the S1* -state localized on the acceptor fragment.
Thus the energy transfer from the donor fragment to the acceptor fragment may be interpreted as the internal conversion process. The constants kic increase by an order of magnitude due to fluorination the donor fragment, while the constants kic decrease somewhat when the acceptor fragment is fluorinated. This fact is correlated with the change in the energy gap between the interacting states [2, 12].
The calculation of geometry of bichromophores 4MU - CH2 - UC17 and 4FMU - CH2 - UC17 has shown, that the fragments can take only mutually perpendicular planes. In the present work the data for TS - CH2 - C120 for this geometry are given, though in earlier work considered are and other conformations for these structures [11, 12]. The analysis of distribution charges has shown, that under excitation in states S1, S2 and S3 in linear bichromophore the transfer of electronic density from the coumarin fragment on stilbene fragment and, mainly, on group CH2 (~ 0,1 e) occurs. Expansion of wave functions of three lower electronic states in terms of one-electronic configurations has shown that for this bichromophore all the states are mixed.
Our investigations have shown [1, 2], that bichromophores are more complex molecular systems, than a mere sum two nonconjugate fluorophores. Bichromophore is a unified system - "supermolecule" with the unified system of MO largely belonging to separate fragments, but on the other hand, unified MO. Two types of the states in the absorption spectra of the test bichromophores have been detected: the excited states localized on the separate fragments (donor or acceptor) and the mixed states with considerable charge transfer. Fluorination of the donor fragment decreases the energy of the mixed states and, consequently, these states may play a more important role in the photoprocesses involved in the bichromophores. The characteristic feature of bichromophore is the fact, that along with the excited states localized on separate bichromophore fragments, there are states, delocalized on the whole molecule, including wave functions of atoms of the donor and acceptor fragments. In bichromophore the different from Förster mechanism for intramolecular transfer energy may be realized. Recognizing the mixed character of the electronic states of the test bichromophores and treating them as a unified molecular system we have assumed the following scheme for photophysical processes in the bichromophore molecules. After optical excitation of the strong donor absorption band there develop fast internal conversion processes with the result that the molecule is found in the S1*-state localized on the acceptor fragment.
Thus the energy transfer from the donor fragment to the acceptor may be interpreted as the internal conversion process. The rate constants of internal conversion (kic) are given in Figures 3-5 . The rate constants kic increase by an order of magnitude due to fluorination the donor fragment. This fact is correlated with the change in the energy gap between the interacting states [2].
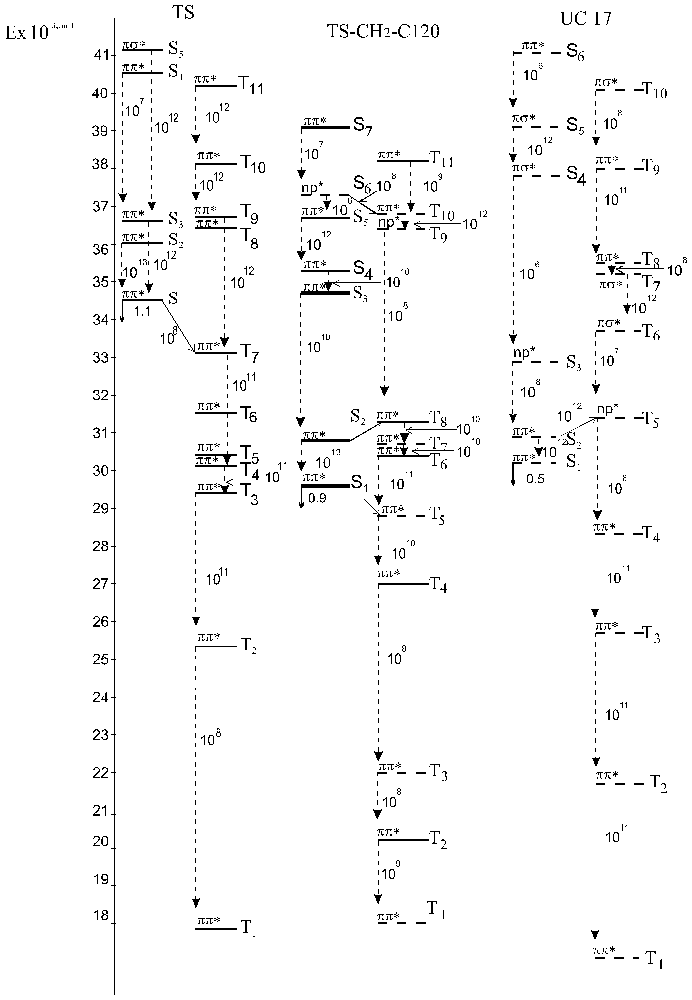
Fig. 3 - Schemes of electronic excited states for TS - CH2 - C 120 and its incoherent molecules calculated by the INDO method: vertical dashed lines for kic and slant solid lines for kST. Bold lines show mixed states.
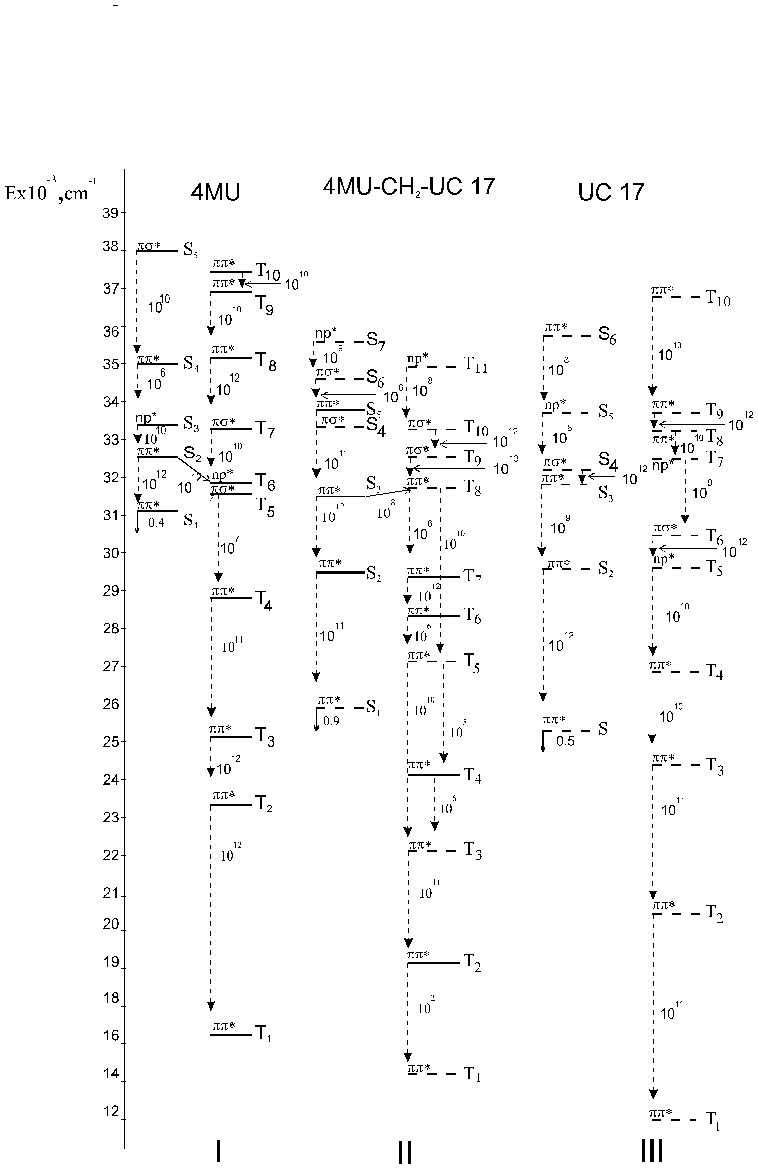
Fig. 4 - Schemes of electronic excited states for 4MU - CH2 - UC17 and its incoherent molecules calculated by the INDO method: vertical dashed lines for kic and slant solid lines for kST. Bold lines show mixed states.
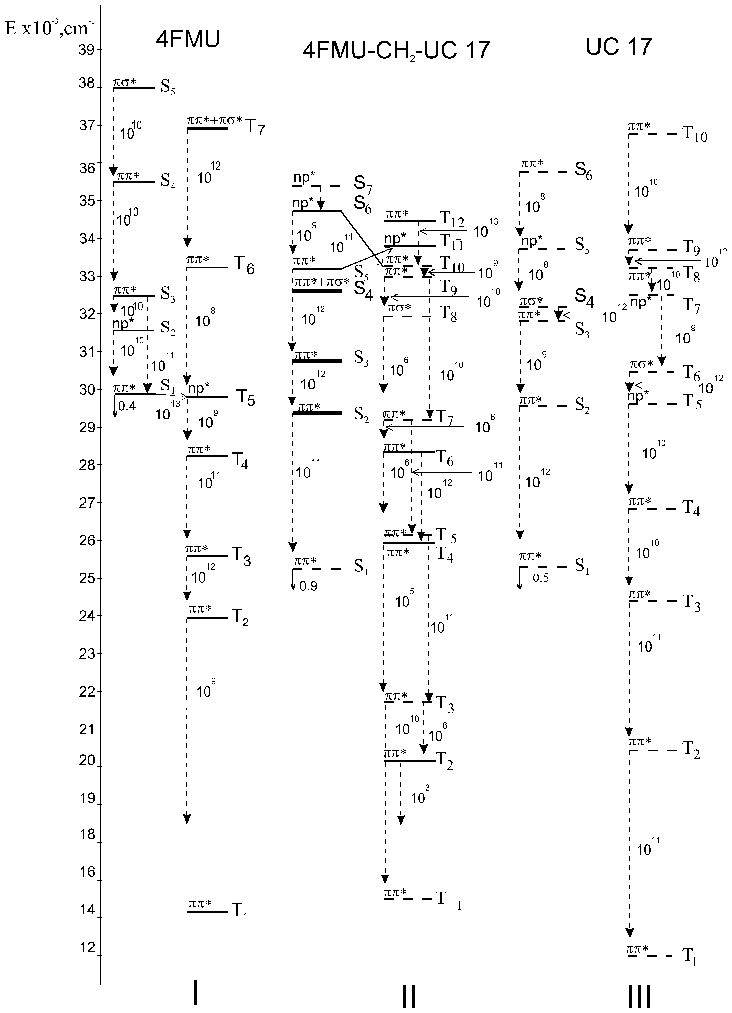
Fig. 5 - Schemes of electronic excited states for 4FMU - CH2 - UC17 and its incoherent molecules calculated by the INDO method: vertical dashed lines for kic and slant solid lines for kST. Bold lines show mixed states.
CONCLUSION
The two types of states in the absorption spectra of the test bifluorophores have been detected: the excited states localized on the fragments (D or A) and the mixed states with considerable charge transfer. Fluorination of the energy donor decreases the energies of the mixed states and, consequently, these states come to play a more important role in the photoprocesses involved in the bichromophores. The analysis of constants of internal and singlet-triplet conversion received in our calculations (Fig. 3-5 ) shows that we may assume the following schemes of photophysical processes in the test bichromophores (Fig. 6.)
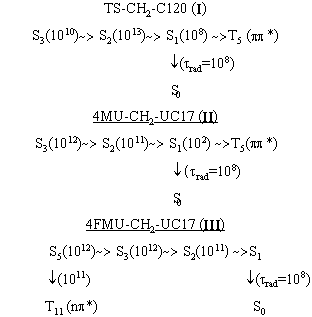
Fig. 6 - Schemes of photophysical processes in the test bichromophores
It is clear, that the process of singlet-triplet conversion competes with the process of radiation energy degradation of a molecule, which may be the reason for decreasing the lasing efficiency of TS-CH2-C120 bichromophore.
In nonlinear bichromophores the lower electron state S1* is located only on the acceptor fragment, and all other states pi-pi* are mixed. In 4MU-CH2-UC17 the radiative channel of the excited energy degradation is shown to be predominant. The fluorescence yield of this bichromophore does not change as compared to that of UC 17. In 4FMU-CH2-UC17 bichromophore the singlet-triplet conversion between S5* and T11* states is possible. In our opinion this channel is able to compete with the process of the internal conversion and it is responsible for the reduced fluorescence efficiency.
ACKNOWLEDGMENTS
We thank researchers Drs. Kropachev A., Il'chenko A. and A. Tolmachyov (Institute of Organic Chemistry by National Academy of Science, Kiev, Ukraine) for synthesis of the test bichromophores.
REFERENCES
- V.Ya. Artyukhov, G.V. Mayer and N.R. Rib (1996). A quantum chemical study of singlet-singlet electronic energy transfer in bifluorophore molecular systems Optics and Spectroscopy, Vol. 81, No. 4, pp. 553-557. Translation from Optika i Spektroskopiya, Vol. 81, No. 4, 1996, pp.607-612.
- N. Vasil'eva, I. Sokolova, L. Samsonova, T. Kopylova, V. Artyukhov, G. Mayer (1995). Photoprocesses in stilbene-based bifluorophores. In Atomic and Molecular Pulsed Lasers. Proc. SPIE Vol. 2619, pp.136-143.
- V.Ya. Artyukhov and A.I. Galeeva (1986). Soviet Physics Journal, Vol. 29, pp. 949-952.
- V.Ya. Artyukhov (1978). Program of calculation of molecular electrostatic potential. Zh. Strukt. Khimii, Vol. 19, No 3, pp. 418-422.
- G.V. Mayer and V.Ya. Artyukhov (1993). Atmospheric and oceanic optics. Vol. 6, No. 6, pp. 278-286.
- G.V. Mayer (1992). Photophysical Processes and Lasing Ability of Aromatic Molecules, TSU, Tomsk.
- R.T. Kuznetsova., T.N. Kopylova, G.V. Mayer, L.G. Samsonova, I.V. Sokolova, V.Ya. Artyukhov, O.K. Bazyl', E.N. Tel'minov and K.M. Degtyarenko (1995). "Effect of stimulated radiation on the photostability of laser dyes", Proceedings of the Intern. Conference on Lasers'94. Canada, STS Press, McLean, V.A., pp.126-133.
- A.N. Terenin (1967). Photonics of Dye Molecules, Nauka, Leningrad.
- K.S. Peters, S.C. Freilich and J. Lee (1992). "Picosecond dynamics of trans-stilbene photodimerization," J. Phys. Chem., Vol. 97, pp. 5482-5485.
- R.J. Sension, S.T. Repinec, A.Z. Szarka, and R.M. Hochstrasser (1993). J. Chem. Phys. Vol. 98, No. 8, pp. 6291-6314.
- I.V. Sokolova, N.Yu. Vasil'eva, Ja.O. Vylegzhanina and G.V. Mayer (1993). Izv. Vyssh. Uchebn. Zaved. Fiz., Vol. 36, pp. 882-886.
- I.V. Sokolova, N.Yu. Vasil'eva, Ja.O. Vylegzhanina and G.V. Mayer (1995). Optika i Spektroskopiya, Vol. 95, No. 3, pp. 460-464.
- V.Ya.Artyukhov, N.Yu.Vasil'eva, Ò.N. Kopylova, R.T. Kuznetsova, G.V.Mayer, N.R. Rib, L.G. Samsonova and I.V. Sokolova (1995). Ultrafast energy transfer in bifluorophores. 15-th Intern. Conference on Coherent and Nonlinear Optics. Techn. Digest. St. Petersburg, Vol. 1, pp. 160-161.