ISOLATED NEURON RESPONSE TO BLUE LASER MICROIRRADIATION: PHENOMENOLOGY AND POSSIBLE MECHANISM
A.B. UZDENSKY
Department of Biophysics and Biocybernetics,
Physical Faculty, Rostov State University,
Stachky av., 194/1, Rostov-on-Don, 344090, Russia
e-mail: uzd@krinc.rnd.runnet.ru
1. Introduction
Wide laser applications in medicine stimulated extensive studies of visible light effects on non-pigmented animal cells which does not contain specific light-absorbing molecules [1-4]. Nerve cells are not the traditional photobiological object. However, the data on the laser light influence on neurons are of importance for laser neurosurgery, therapy, hygiene, ophthalmology and other fields of laser medicine where nerve tissue undergoes intensive laser impact. Moreover, the study of laser irradiation effects on nerve cells can give some information on the common photobiological mechanisms of the laser radiation effects on non-pigmented animal cells and help in study of some neurophysiological problems including the relationships between cell function , structure, and metabolism.
Since the pioneer works of N. Chalazonitis and A.Arvanitaki it is well known that visible light changes electrophysiological processes in invertebrate pigmented neurons. Illumination can inhibit or excite them depending on the pigment nature in these cells [5-9].
Earlier non-pigmented neurons were considered as non-sensitive to visible light [10]. However, shortly after the development of lasers their photosensitivity was established. It has been shown that visible laser light can effectively influence bioelectric processes [11-17], ultrastructure [14,16], and metabolism in nerve cells [14]. But the phenomenology and mechanisms of nerve cell response to laser irradiation, and their dependence on laser light parameters such as wavelength, intensity, exposure, irradiation mode, etc. have not been sufficiently studied. The majority of authors investigated neuron alterations after short laser pulses, but prolonged effects of continuous laser irradiation leading to nerve cell death were unstudied. However, it is of importance to study the dynamics of neuron responses in the course of irradiation.
The goal of the present paper was the review our data on the dynamics of the functional, structural , and metabolic changes in a single non-pigmented invertebrate neuron under blue laser microirradiation. We tried to elucidate some mechanisms underlying the different phases of the cell responses and assumed that the similar processes take part in the responses of different animal non-pigmented cells under the same irradiation.
2. Materials and methods
2.1. Cells
A suitable object for the study of the problems outlined is a crayfish stretch receptor neuron (SRN): a large single nerve cell (Fig.1) capable to prolonged firing with a nearly constant rate during several hours. At this stable background one can study continuously and precisely (with the accuracy about 3-5 %) initial threshold changes of neuronal membrane state, dynamics of bioelectric, metabolic, and structural changes, and terminal events leading to the cell death. SRN is a classic neurophysiological object, and its structure, electrophysiological and biochemical features are well studied [18,19 ].
![]()

Fig.1. Slowly adapting crayfish stretch receptor neuron. Cytochemical staining for cytochrome C oxidase. Ob. 10x.
![]()
In our experiments slowly adapting muscle receptor organs of the crayfish Astacus leptodacilus were isolated as described by Wiersma et al. [20]. They were placed into a plexiglas chamber equipped with the device for receptor muscle extension and filled with van Harreveld saline (mM: NaCl - 205; KCL -5,4; NaHCO3 - 0,24; MgCl2 - 5,4; CaCl2 - 13,5; pH 7,2-7,4). Stretch receptor neurons were capable of regular firing during 10-15 hours in this conditions. The majority of experiments were carried out in summer at the temperature range 21 +/- 3 øC.
2.2. Electrophysiological recording
Neuron spikes were derived extracellularly from axons by the silver wire or pipette suction electrodes and amplified by the amplifier UBP2-03 (USSR). Their frequency was converted into voltage by the analog frequency meter (made in our laboratory and calibrated using a pulse generator (G6-27, USSR)), and continuously recorded by a chart-recorder (N-390, USSR). An oscilloscope (S1-69, USSR) was used for spikes and laser pulses visualization. At the beginning of each experiment we adjusted the initial neuron firing level to 10-15 Hz by an appropriate receptor muscle extension.
2.3. Laser irradiation
The laser microirradiation teqnique was used in our research. This method invented by S Chakhotin [21] and developed by M.W. Berns, C. Salet, and their collaborators [22-26]. They used lasers as a light sourse allowing damage single mitochondria in myocardial cells, and to study the following changes in cell beating, their ultrastructure etc.
The experimental protocol was as follows: after a stable control spike generation during 15-30 min various neuron regions were irradiated with a 5-8 mm laser microbeam (Fig.2). It was focused by the microscope Biolam R1 (USSR) equipped with an opaque-illuminator with a half-transparent silver mirror. The latter directed the laser beam into the microscope optic system. The vertical section of the laser microbeam (Fig.1,A) and minimal beam diameter were determined with the help of calibrated ocular micrometer MOV-15 (USSR) and microscope supply scale (vertical dimension). Taking into account the light energy losses in the optic system, the mean laser microbeam intensity was calculated Wrom: TWFA
I = 4 a*b*P/p*d 2
where P is the input mean laser beam power, d - the minimal microbeam diameter, a - the reflectance of the opaque-illuminator, and b - the objective transmittance. It varied from 101 to 1.5*104 W/cm2 in the case of He-Cd laser irradiation; from 2*103 to 2*104 W/cm2 in the case of He-Ne laser irradiation, and from 0.25 to 5*103 W/cm2 in the case of dye laser irradiation. The peak intensities in the last case varied from 0.4*108 to 8*108 W/cm2. The laser radiation sources used were: the helium-cadmium laser LG-62 (USSR, 441.6 nm, 120 mW) used in the majority of experiments, the helium-neon laser LG-36 (USSR, 632,8 nm, 30 mW) or the dye laser Spectrolas-3 (Jobin Yvon, France, spectral range from 434 to 600 nm) pumped by the nitrogen laser LGI-501 (USSR , pulse duration 15 nsec, repetition rate 400 Hz). Mean irradiation power was measured by the laser dosimeter IMO-2N (USSR). The same mean intensity 1.25*103 W/cm2 was set for each wavelength in action spectra studies. The irradiation exposure was usually long sufficiently to reveal all neuron response stages until the irreversible firing cessation. Neuron firing frequency was recorded continuously in the cource of its response to laser irradiation.
![]()
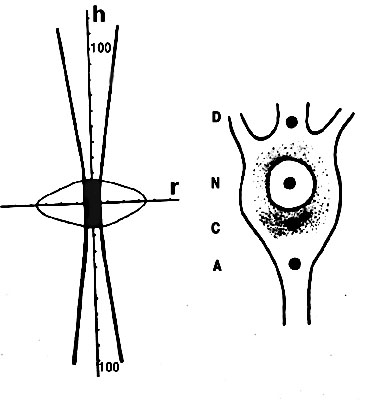
Fig.2. The scheme of the laser beam focusing. Left - the vertical section of the microbeam, right - microirradiated neuron regions: A - axon, an initial segment, C - cytoplasm between nucleus and axon, N - nucleus, D - dendrite, an initial segment.
![]()
In the majority of the experiments neuron cytoplasm between axon and nucleus (C-region, abundant with mitochondria and most sensitive to laser microirradiation) was irradiated. In some cases other cell regions were irradiated (Fig.1,B): axon (A-region), nucleus (N-region), or dendrite (D-region).
2.4. Neuron response modification
Neuron responses to a laser microirradiation were modified by the bioenergetic inhibitors: cytochrome oxidase inhibitor sodium azide (2-5 mM), oxidative phosphorylation uncoupler 2,4-dinitrophenol (0.1 mM), or glycolysis inhibitor sodium monoiodacetate (0.5-1 mM); by anti-radical agents: triplet state scavenger sodium iodide (10-20 mM), antioxidant dibunol (obtained from Prof. E.B. Burlakova, Institute of Chemical Physics, Moscow , 0.02 mM), and OH. -radicals scavenger D-mannit (10-100 mM); by 1/3 or 3-fold Ca concentrations relatively the normal Ca level in the saline; by supravital stains: Janus Green B (Merk, FRG, 7*10-6 g/ml), and sodium fluorescein (10-6 g/ml).
All reagents were manufactured in the USSR if the manufacturer is not indicated. Modificator concentrations used did not change themselves neuron firing significantly. After 20 min control exposure in the saline with the modificator added , the modified cells were irradiated, and the parameters of neuron response dynamics were recorded and compared with the unmodified ones.
2.5.Transmission electron microscopy
Neuron ultrastructure was studied at the different phases of its bioelectric response to He-Cd laser microirradiation (441.6 nm, 103 W/cm2) of the C-region. With this aim at the appropriate electrophysiologically monitored moments cells were fixed 20 min with 2.5 % glutaraldehyde in 0.1 M phosphate buffer, pH 7.2. Then neurons were cut out together with the 1-2 mm pieces of receptor muscle so that the preparations were T-shaped. Then preparations were washed in phosphate buffer, osmicated 1 hour in 1% OsO4, contrasted with uranyl acetate, dehydrated in a graded series of ethanol up to 100 %, and embedded in epoxy resin. Ultrathin sections were stained with lead citrate and viewed in a BS-242E electron microscope.
2.6. Cytochemical study of succinate dehydrogenase activity
As in the case of electron microscopic study the succinate dehydrogenase activity was studied at the different phases of its bioelectric response to He-Cd laser microirradiation (441.6 nm, 103 W/cm2) of the C-region. At the appropriate moments of cell response to irradiation the saline in the experimental chamber was quickly changed by the following incubation mixture: sodium succinate, 0.05 mM; nitrotetrasolium blue (Chemapol, Chehoslovakia), 1 mg/ml; magnesium chloride, 5 mM; sodium azide, 10 mM; 50 mM tris-HCl buffer, pH 7.2. After washing preparation postfixed in 2 % glutaraldehyde and embedded in glycerol-gelatin [27]. Cell were photographed on a microscope MBI-6 and negatives were photometered using a scanning microphotometer IFO-451 (USSR).
2.7. Biochemical ATPase assay
Rat brain tissue after decapitation was homogenized at +4o C in tris-HCl
buffer (0.02 M, pH 7.5) and protein content was determined by the Lowry method. Then
homogenate aliquotes were irradiated by the argon laser (488 nm, 0.1 W/cm2)
during 1, 10, 100 or 1000 sec (doses 0.1, 1, 10 or 100 J/cm2, respectively).
Control probes were not irradiated. Then samples were quickly frozen and after unfreezing
ATPase activity was determined. Total ATPase activity was assayed from inorganic phosphate
increase during 20 min incubation at 37o C in the medium containing (mM):
tris-HCl buffer, pH 7.4 - 30, disodium ATP - 3, NaCl - 100, KCl - 20, MgCl2 -
5, EDTA - 0.2. Mg2+-ATPase activity was determined in this medium without Na
(120 mM KCl were added to match the solution ionic strength). Activity of Mg2+-dependent
(Na+-K+)-ATPase was calculated from the difference between the total
and Mg2+-ATPase activities. Ca2+-ATPase activity was also determined
as the difference between the total and Mg2+-ATPase activities, however the
total ATPase activity was assayed in the following medium (mM): tris-HCl buffer , pH 7.4 -
30, CaCl2 - 0.05, KCl - 120, MgCl2 - 5, disodium ATP - 3. Mg2+-ATPase
was assayed in the same medium in the presence of 1 mM EGTA. Reactions were stopped by
adding 0.4 ml of 20 % trichloracetic acid. Inorganic phosphate was assayed by
spectrophotometric measuring of 390 nm absorption of molibdate complexes formed in
phosphate reaction with ammonium molibdate [28]. Enzyme activity was expressed in ![]() of Pi produced for 20 min per 1 mg of
protein.
of Pi produced for 20 min per 1 mg of
protein.
2.8. Statistics
Standard statistical methods based on the Student's t-test were used including correlation and regression analysis and nonparametric Wilkokson’s test [29]. Data are expressed as means ± 95 % confidence intervals.
3. Single neuron response to blue laser microirradiation
3.1 Neuron response dynamics
Firing of a single non-pigmented nerve cell was insensitive to red laser radiation, but changed under blue laser irradiation [30,31]. In the majority of experiments SRNs responded to prolonged blue laser microirradiation polyphasically: after some latency (L) firing frequency increased (phase I), then decreased gradually (phase II), increased again (phase III), and after reaching a relatively high level (30-50 Hz) neuron firing ceased irreversibly and abruptly (phase IY). For the quantitative analysis we characterized every response phase with the set of the following parameters: durations, ti, values of impulse frequency changes, D fi, and rates of firing acceleration or inhibition, vi, , where "i" is the phase number (Fig.3) [30,31].
In the certain experimental conditions this EIE-type (excitatory-inhibitory-excitatory) of neuron response dynamics was modified: its different phases were more prominent, or reduced, or absent. In some cases the excitatory response (E-type) with a spike cessation immediately after the phase I was observed. Such E-type responses were recorded mostly in winter, whereas full EIE-responses were observed usually in summer and autumn. Sometimes neuron response consisted of the excitatory phase followed by the inhibitory one (EI-type). In this case the inhibition process (phase II) was so strong that neuron could not resume firing. This inhibition phase was presumably due to neuron hyperpolarization because this cell retained spike generation ability and fired under an additional receptor muscle extension which generated an additional depolarizing receptor potential [8]. However, sometimes cell lost excitability irreversibly.
One can assume that the complex EIE-type dynamics is the superposition of two oppositely directed processes: (i) depolarization causing firing acceleration, and (2) hyperpolarization developing some later (Fig.4). Inhibitory resources inducing inhibition process in the phase II are thought to be limited, and continuously acting excitatory factors may become dominant again after the exhaustion of inhibitory factors and accelerate neuron firing repeatedly. To prove this mechanism we modified neuron response to laser microirradiation with different chemicals and studied response dynamics changes. Experimental data are summarized in the Table 1.
![]()
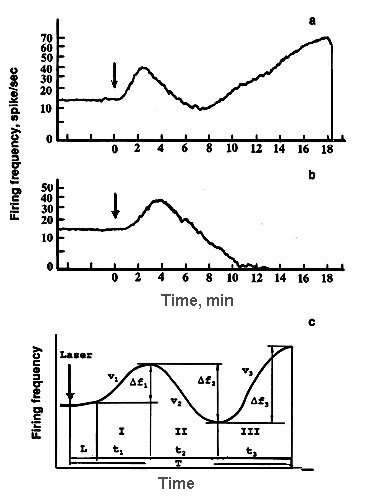
Figure.3. (a) EIE-type of a single neuron response dynamics to laser microirradiation and (b) EI-type. (c ) Schematic representation of neuron response to laser microirradiation and the designations used. Ordinate - firing frequency, abscissa - time.
![]()
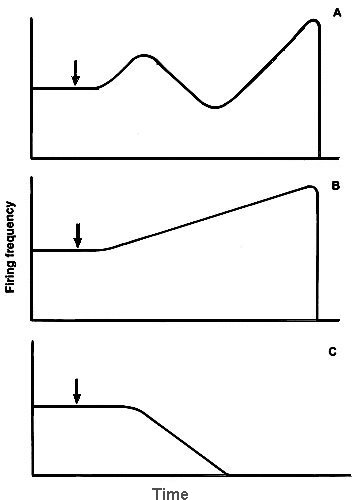
Figure 4. Hypothetic scheme of the mechanism of neuron respose dynamics: the complex EIE response (A) is assumed to be the superposition of excitatory firing changes (B) and inhibitory ones (C).
![]()
TABLE 1. Modifications changing different phases of neuron response to laser microirradiation of its cytoplasm region
| Modifications | Response types |
Latency | Phase I |
Phase II |
Phase III |
Lifetime | Reference |
| 2,4-dinitrophenol: N-region C-region |
E or EIE EIE |
|
|
|
|
|
31,32 |
| Sodium fluorescein | EIE | 31,32 | |||||
| Janus green B N-region C-region |
E EIE |
|
|
absent
|
absent
|
|
31,32 |
| Sodium monoiodacetate |
E | absent | absent | 31,32 | |||
| Sodium azide | E | absent | absent | 31,32 | |||
| 1/3 [Ca] saline | E | absent | absent | 31,32 | |||
| 3 [Ca] saline | EI | absent | 31,32 | ||||
| Dibnol | E | n.d. | n.d. | 33 | |||
| D-mannit | EIE | 33 | |||||
| Sodium iodide | EI | absent | 33 |
Types of the cell response: EIE - excitation-inhibition-excitation, EI -
excitation-inhibition, E - excitation . ![]() - no significant changes;
- no significant changes;![]() - increase of parameters describing the appropriate response phase
(p<0.05);
- increase of parameters describing the appropriate response phase
(p<0.05); ![]() - decrease of parameters describing the appropriate response phase (p<0.05);
n.d. - no data. The only significant changes (p<0.05) are presented.
- decrease of parameters describing the appropriate response phase (p<0.05);
n.d. - no data. The only significant changes (p<0.05) are presented.
![]()
3.2. Action spectra
The study of action spectra of neuron response to laser microirradiation carried out in
the collaboration with V.V.Savransky [31,34]. Pulse-periodic laser irradiation with a mean
intensity of 1.25*10![]() W/cm
W/cm![]() and different wavelengths in the blue spectral
range from 434 to 500 nm caused the same neuron responses of EI-type. Neuron irradiation
at the same power density and with longer wavelengths: 580, 600, and 632.8 nm was not
effective. Action spectra of neuron response to C-region microirradiation revealed the
rather sharp maximum at 460 nm for the phase I parameters: L, t1 and v1.
Absorption spectra with this maxims are known to be characteristic for flavin compounds,
and we suspected flavins as the primary photoreceptors in the neuron. Action spectra of
the phase II parameters: t2 and v2 were not so sharp and contained
additional maximum at 488 nm. Hence some additional component (presumably carotenoids)
participated in the phase II occurring (Fig.5). Thus the blue light was the most
efficient. In the majority of the following experiments we studied the effects of blue
radiation of He-Cd laser (441.6 nm).
and different wavelengths in the blue spectral
range from 434 to 500 nm caused the same neuron responses of EI-type. Neuron irradiation
at the same power density and with longer wavelengths: 580, 600, and 632.8 nm was not
effective. Action spectra of neuron response to C-region microirradiation revealed the
rather sharp maximum at 460 nm for the phase I parameters: L, t1 and v1.
Absorption spectra with this maxims are known to be characteristic for flavin compounds,
and we suspected flavins as the primary photoreceptors in the neuron. Action spectra of
the phase II parameters: t2 and v2 were not so sharp and contained
additional maximum at 488 nm. Hence some additional component (presumably carotenoids)
participated in the phase II occurring (Fig.5). Thus the blue light was the most
efficient. In the majority of the following experiments we studied the effects of blue
radiation of He-Cd laser (441.6 nm).
3.3 The dependence of SRN response dynamics on microirradiation intensity
The decrease of 441.6 nm microirradiation intensity elongated the neuron response: rates of firing changes in all phases vi decreased, and temporal parameters - latency L, different phase durations ti, and neuron lifetime T increased (Fig.6). The intensity dependencies of these parameters were well approximated with the power function: P = a*Ik, where P is a studied parameter, I - microirradiation intensity, a - constant, and k - exponent determined by the least-square method (Table 2) [30, 31, 34].
![]()
Table 2. Values of the exponent k for different parameters of SRN response to laser microirradiation (441.6 nm) versus irradiation intensity
| Irradiation regime |
L | T | V1 | V2 | V3 |
| Continuous | -0.6 | -0.6 | +0.6 | +0.2 | +0.4 |
| Pulse-periodic | -1.5 | - | +2.1 | +1.0 | +1.9 |
In the case of continuous irradiation k < 1 for all parameters. It means that all response phases were not induced by the linear photochemical processes only but some secondary dark reaction contributed to the cell response. It is of interest that the value k=0.2 for the inhibition process (for v2) was less than for the excitation ones (for v1 and v3) showing that some secondary reactions made the larger contributions in this process.
In the experiments with a pulse-periodic microirradiation (Fig.6,B) [34] different dependencies were observed for different neuron response parameters. So, the firing acceleration rates v1 and v3 in the phases I and III, depended quadratically on irradiation intensity: k = 2.1 and 1.9, respectively. This suggests that two-photon processes were involved in reactions leading to the firing stimulation in these phases. The firing inhibition rate v2 in the phase II depended linearly on irradiation intensity as a monophotonic reaction.
![]()
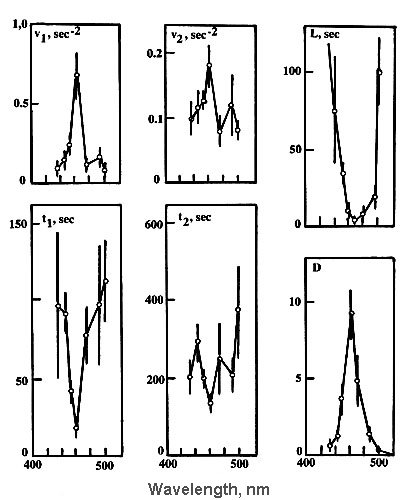
Fig.5. Wavelength dependencies for different parameters (see Fig.3,c) describing
dynamics of neuron responses to laser microirradiation. The mean laser beam intensity was
1.25*10![]() W/cm
W/cm![]() . Mean values and 95 % confidence intervals are shown.
. Mean values and 95 % confidence intervals are shown.
![]()
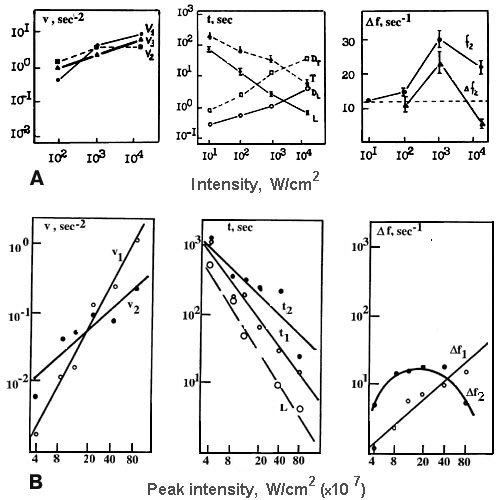
Fig.6. Laser microbeam intensity dependencies for parameters describing dynamics of
neuron responses (see Fig.3,c) to laser microirradiation (441.6 nm). (A) - continuous
irradiation, (B) - pulse-periodic irradiation (to obtain the peak or mean laser beam
intensities the abscissae scale in B have to be multiplied by 0.625*10![]() W/cm
W/cm![]() ).
).
![]()
3.4 Accumulation of irreversible photo-induced changes in the neuron
The response latency could be as long as 60-90 min at the lowering of microirradiation intensity to 10 W/cm2. This showed that some irreversible subthreshold changes were accumulated in the cell during such prolonged latent period and caused firing shifts after an hour or more. Experiments with fractionated irradiation demonstrated that preliminary short microirradiation (441.6 nm) sensitized SRN to the following prolonged impact: it shortened response latency L and neuron lifetime T, and accelerated firing changes in different response phases (Fig.7). Even preliminary subthreshold exposures (less than L) which could not change SRN firing themselves changed neuron response to the consecutive laser impact. The longer exposures could change qualitatively the response type from EIE to E and intensify all response phases. Hence some perturbations which could not be abolished during the long latency or period between two consecutive irradiations were accumulated in the cell under the blue laser microirradiation.
3.5 . The dependence on irradiation localization
The first indications to the possible laser microirradiation targets in SRN were obtained in the experiment with microirradiation of different neuron regions [24,25]. It was shown that the C-region, rich in mitochondria, is most sensitive to laser microirradiation. Two different kinds of the dependence of neuron response dynamics on the irradiation place were observed (Table. 3). In the first one the parameter v3 describing the excitatory phase III was reduced when the irradiation point moved from axon to dendrites and the neuron lifetime T was increased. Neuronal membrane excitability is also reduced in this direction, and we suspected that the laser effect on neuronal membrane was the reason of the firing acceleration phases. In another case the inhibitory phase II was most prominent under the irradiation of C-region abundant with mitochondria as compared to dendrite or axon microirradiation. Because the light absorbing molecules such as cytochromes and flavins are concentrated in mitochondria, we assumed that this phase II was the result of laser effect on mitochondria.
![]()
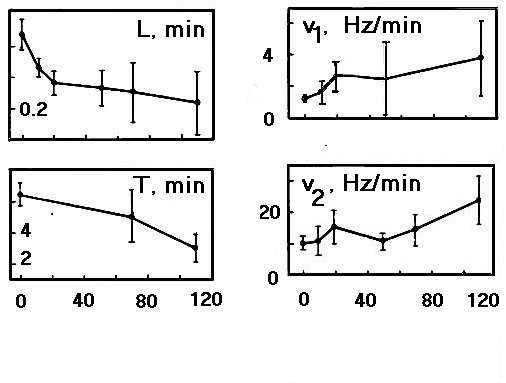
Fig.7. The dependencies of parameters describing dynamics of neuron responses (see Fig.3,c) on the duration (sec) of preliminary laser microirradiation (441.6 nm, 1.5*104 W/cm2.
![]()
Table 3. Relative values of parameters characterizing different phases of the neuron responses to 441,6 nm laser microirradiation of its different regions normalized to mean values for all irradiated sites, all intensities and all exposures
| R Response parameters |
A | C | N | D | Pairs of significantly different parameters |
| L | 1.1 | 0.9 | 0.9 | 1.1 | A-C, A-N, D-C, D-N |
| v1 | 1.0 | 1.1 | 1.0 | 0.9 | D-C |
| v2 | 1.0 | 1.3 | 1.1 | 0.7 | D-C |
| v3 | 1.15 | 1.15 | 0.85 | 0.75 | A-N, A-D, C-N, C-D |
| T | 0.8 | 1.0 | 1.05 | 1.15 | A-D |
![]()
3.6. Neuron photosensitization
Specific photosensitization of different cellular structures with supravital stains significantly increases the selectivity of laser action [31,32]. Photosensitization of neuronal membrane with sodium fluorescein (10-6 g/ml) which adsorbing at the cellular surface and not penetrating into the cell due to its negative charge (it was proved by fluorescent microscopy), caused 5-fold decrease of latency L and lifetime T, and 8- and 2-fold increase of frequency acceleration rates v1 and v3, respectively. Meantime, the phase II was reduced and inhibition rate v2 was decreased by 1.5 times (Table 1) [32]. These data support the assumption that the firing acceleration phases I and III were caused by the laser radiation effect on the neuronal membrane.
Janus green B selectively staining mitochondria in living cells [35] increased markedly the photosensitivity of non-pigmented neurons (Table 1). Even the red helium-neon laser radiation, which could not change nerve cell firing itself, influenced effectively the stained SRN firing. Focusing the laser microbeam into the nucleus we observed the E-type responses in the most of experiments while the EIE responses were observed under C-region irradiation [31,32]. Considering the vertical section of laser microbeam focused into the different intracellular sites one can see that light flux through the neuronal membrane was almost the same at both irradiation locations. However, the number of irradiated mitochondria at the microbeam focusing into the nucleus center was significantly less as compared to C-region focusing.
Similar difference between the results of blue laser microirradiation of C- and N-regions was observed in the case of SRN response modification with 2,4-dinitrophenol, a known uncoupler of oxidative phosphorilation in mitochondria [31,32]. However, at the concentration used (0.1 mM) it did not shift the neuron firing level itself. It seems that this yellow substance well absorbing the blue light sensitized SRN to laser radiation due to the photodynamic effect. The combined action of 2,4-dinitrophenol and laser microirradiation (441.6 nm) vastly intensified the firing acceleration in the phase I (Fig.8). In the cases of C- or N-region microirradiations the acceleration rate v1 was approximately the same. However, the inhibition phase was weak at the N-region irradiation , but very prominent under the C-region impact (Table 1). Hence, the phase II was related with laser-induced mitochondria perturbations.
3.7. Bioenergetic inhibitors
Using inhibitors acting selectively on different components of the bioenergetic chain one can change their redox-states and hence their optical properties. Both, sodium azide inhibiting cytochrome C oxidase, the terminal point of respiration chain,
![]()
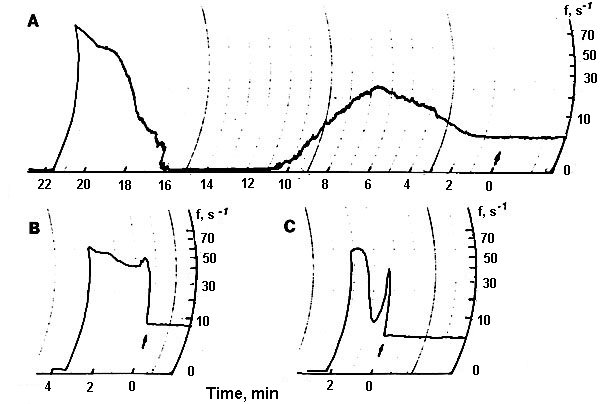
Fig.8. 2,4-dinitrophenol ( 0.1 mM ) sensitization of neuron response to laser microirradiation (441.6 nm, 103 W/cm2) . (A) Neuron response without sensitization, (B) N-region irradiation of sensitized neuron, (C) C-region irradiation of sensitized neuron. Ordinate - firing frequency , Hz, abscissa - time, min. The arrow shows the irradiation onset.
![]()
and the glycolytic inhibitor sodium monoiodacetate preventing the electron supply to initial points of the electron transfer chain produced polyphasic changes of neuron firing at the concentrations > 10 and 4 mM, respectively. The dynamics of these changes resembled the laser-induced neuron response: rapid increase of firing frequency (without latency), then decrease, repeated increase and abrupt cessation of spike generation. This similarity suggests that points of action of different bioenergetic inhibitors and blue laser radiation were the same, and bioenergetic processes were involved in the neuron response to laser microirradiation. At the less concentrations sodium azide (2-5 mM) or sodium monoiodacetate (0,5-1 mM) did not change neuron firing themselves but modified its response to the following laser microirradiation. Both inhibitors caused the shortening of response latency and reduced or eliminated the inhibition phase II in the majority of the experiments (Table 1) [31,32]. The similarity of the effects of both inhibitors differing in their influence on redox states of electron transfer chain components indicated that this difference and consequently the difference in optical absorption of the cell was not very significant for the phase II elimination. However, the inhibition of energy production was more important.
3.8. Electron-microscopic observations
Electron-microscopic experiments carried out in the collaboration with G.M.Fedorenko [36] showed that the blue laser microirradiation (441.6 nm, 103 W/cm2) of neuron cytoplasm affected mitochondria more significantly than other organelles. In the irradiated cytoplasm region mitochondria swelling, disruption of cristae and partial or complete loss of matrix were observed (Fig.9). Inner mitochondrial membranes containing light-absorbing redox-systems were much more damaged than outer ones (Fig.9D). The degree of mitochondrial lesion depended on the phase of neuron response to laser irradiation [31,36]. In the non-irradiated neuron areas mitochondrial membranes looked unaffected with well developed cristae and moderately dense matrix (Fig.9C). However, some overall intracellular changes were observed in addition to the local damages. They included: formation of myelin-like bodies which were probably the product of mitochondria degeneration (Fig.9B and C); decrease of Golgi number, protrusions and wrinkles of nuclear envelope directed to the injured cytoplasm region (Fig.9D). These effects were evidently the secondary ones.
![]()
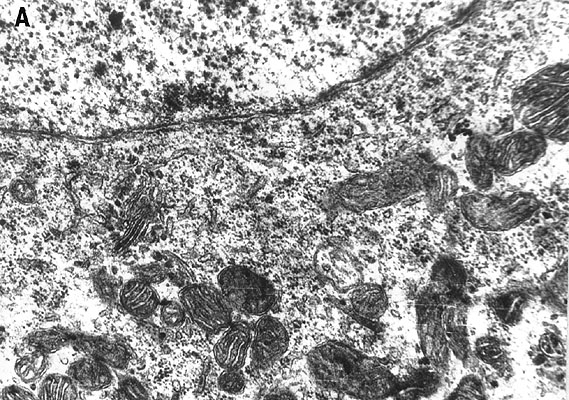
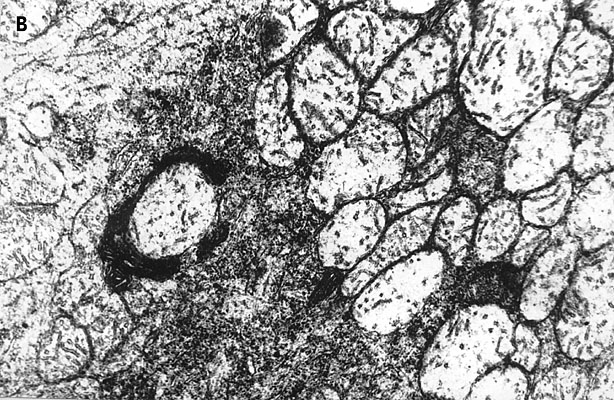
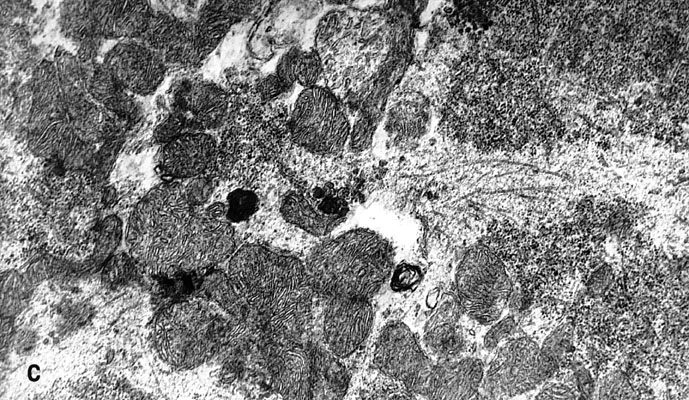
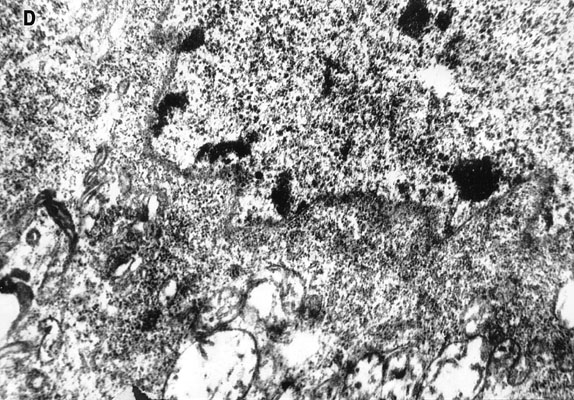
Fig.9. Electron micrographs of the microirradiated neuron (441.6 nm, 103 W/cm2). (A) Control: unirradiated cell. (B) Phase II of the neuron response (See Fig.3C). Irradiated C-region . ( C ) The same cell, unirradiated region in the dendrite zone. (D) Phase III of the neuron response (See Fig.3C). The part of the irradiated region in the C-zone.
![]()
3.9. Cytochemical study
What molecular components did serve as the blue light photoreceptors in a non-pigmented nerve cell? Cytochemical study revealed the drastic local inactivation of mitochondrial flavoprotein succinate dehydrogenase in the irradiated C-region of SNR (Fig.10) [37]. At the same time, cytochrome C oxidase, the electron-transferring hemoprotein integrated into inner mitochondrial membrane as well, was insensitive to 441.6 nm laser radiation [31]. This data confirmed that flavins are the possible intracellular photoreceptors of blue laser radiation in a non-pigmented animal cell. The similar data were obtained in the biochemical study [38]: flavins but not cytochromes were responsible for blue light-induced (400-500 nm) inhibition of oxidative phosphorylation in mitochondria. The local effect of SRN microirradiation was accompanied with the overall cell changes including intracellular redistribution of succinate dehydrohenase activity: its relative increase in the non-irradiated dendrite zone (between nucleus and dendrites) as compared to the microirradiated cytoplasmic part situated between nucleus and axon.
![]()
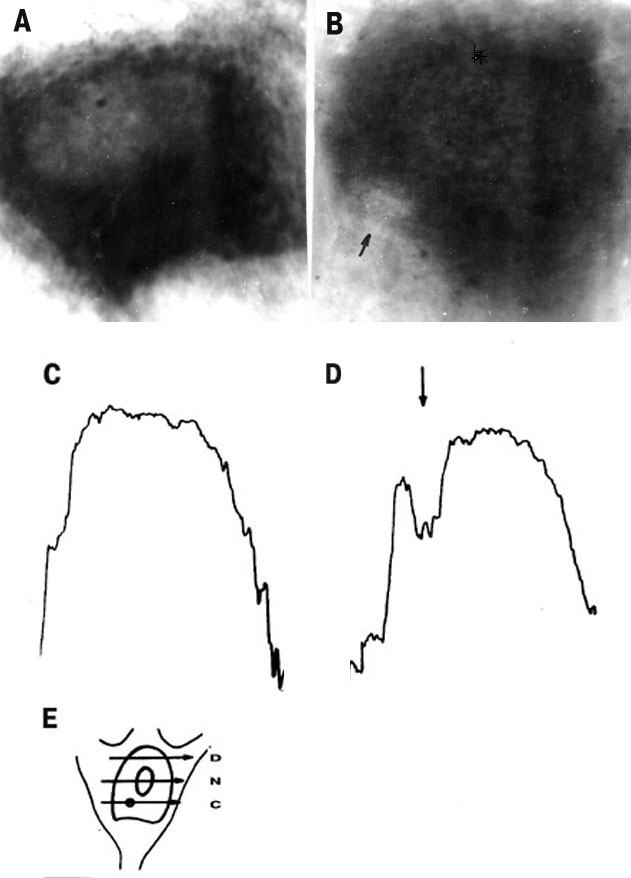
Fig.10. The succinate dehydrogenase activity in the unirradiated (A) and microirradiated (441.6 nm, 103 W/cm2) neurons (B). The arrow on (B) shows the irradiated region in the C-zone of the cell. The optical density distribution along the scanning line C (see the scheme E): in unirradiated cell (C ) and in irradiated cell (D) .
![]()
3.10. Neuron response modification with antiradical inhibitor
In order to study the role of some primary photochemical processes in neuron response to laser microirradiation we modified it with different quenchers of free radical products. It is known that flavin photooxidation produces superoxide anion and hydrogen peroxide [39] promoting free radical lipid peroxidation and destruction of biomembranes [40]. In our experiments antioxidant dibunol (0.02 mM) protected SRN against the blue laser radiation injury [33]. It slowed down acceleration phase I and increased neuron lifetime (Table 1). This shows that the firing acceleration was induced by a free radical lipid peroxidation. (This experiment was carried out in winter when phase II was eliminated probably due to seasonal shifts in animal metabolism, and we could not say anything on the involvement of free radical peroxidation in the inhibitory process. )
Hydroxyl radicals are known as the very active intermediate products in ionizing radiation cell injury [41]. In our attempt to reveal the possible role of hydroxyl radicals in neuron response to blue laser microirradiation using their quencher D-mannit (10 mM) [42] we did not observed significant changes of different response phases (Table 1) [33]. It seems that hydroxyl radicals, very important in radiobiological tissue injury, were not significant in blue laser radiation effect on neuron firing.
Observation of the flavin-like action spectrum forced us to modify the neuron response to laser microirradiation with iodide-anion (10 mM) - a known flavin triplet quencher [43]. It increased the firing inhibition phase II so that a prominent EI-responses were observed in one-half of experiments, and thus elongated the neuron lifetime by 1.4 times [33].
3.11. Laser radiation influence on the brain ATPase activity
Blue argon laser irradiation (488 nm, 0.1 W/cm2) inhibited rat brain (Na+-K+)- and especially Ca2+-ATPase in a dose-dependent manner (Fig.11). (Na+-K+)-ATPase was significantly inhibited to 2/3 of the control level at the doses of 10 and 100 J/cm2. 0.1-1 J/cm2 irradiation caused approximately 2-fold decrease of Ca2+-ATPase activity. Doses 10 and 100 J/cm2 hibited this enzyme almost entirely. Mg2+-ATPase activity decreased under the 0.1 J/cm2 irradiation, but the further dose increase did not change its activity significantly.
![]()
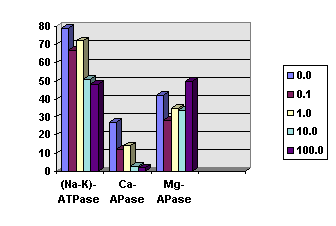
Fig.11. Blue laser light (488 nm) influence on different ATPase activities in rat brain homogenate. Different bars represent various irradiation doses (J/cm2 , see insertion).
3.12. On the Ca2+ participation in the SRN response to laser microirradiation
How do mitochondrial damage evoke the inhibition of neuron activity? One can assume that some mediator molecules releasing from damaged mitochondria diffuse to the cellular membrane and hyperpolarize it. We suggested that Ca2+ ions were most preferable for this role. It is well known that they are actively accumulated in mitochondria and released after mitochondrial lesion [44]. They may hyperpolarize neurons and inhibit their firing through the increase of membrane potassium conductance and the spike generation threshold [18,45,46]. In the experiments with 3-fold calcium concentration in the saline the frequency shifts in the acceleration phase I were reduced but the inhibition phase II was greatly enhanced (Table 1). In this case we observed EI-type response with the phase III eliminated. On the contrary, 1/3-fold calcium concentration markedly increased the neuron excitability. In these conditions the phase I was nearly twice intensive as compared to the normal calcium concentration, the inhibition phase II was eliminated, and we observed the E-type response in almost all experiments (Table I). Hence, calcium ions were necessary for the inhibition phase II [31,32]. It seems, that after the laser-induced mitochondria injury Ca2+released from the lesioned mitochondria, diffused to the neuronal membrane, and inhibited firing. This suggestion is also supported by our finding that the blue laser radiation (488 nm) inactivated Ca2+-ATPase.
![]()
4. On the possible mechanism of the single non-pigmented neuron response to a blue laser microirradiation
Collecting the data obtained we may propose the following mechanism of the blue laser radiation effect on a single nerve cell. This mechanism was probably photochemical but not thermal because of the prolonged latency of firing changes (minutes and tenth of minutes at low irradiation intensities) and the lack of coagulation material at electron-microscopic photographs. It seems that laser radiation absorbed mainly by flavins bound to the cellular membranes damaged them through free radicals formation and lipid peroxidation. Cytochrome-flavoprotein redox chains were found not only in mitochondrial membranes but in other cellular membranes including plasma membrane as well [47]. According to W.Schmidt: considering the primary physiological action of blue light [39]: "a uniform picture appears to crystallize concerning the primary reactions: (1) The photoreceptor pigment is a flavin, probably a flavoprotein, (2) the photoreceptor is bound to a membrane, often the plasmamembrane, in a highly dichroic manner, and (3) the primary reaction is a redox reaction". It is likely that flavins bound to the neuronal membrane were the primary blue light photoreceptors which caused membrane lesions, depolarization, and firing acceleration in the single non-pigmented crayfish neuron under blue laser microirradiation.
Some later the irradiation injured the inner mitochondrial membranes, and free calcium ions presumably released from the lesioned mitochondria, diffused to the neuronal membrane and hyperpolarized it increasing membrane potassium conductance. This caused firing inhibition [18,45,46]. This inhibition process overcame the excitatory effect of the direct neuronal membrane damage and induced the firing slowing down. After exhaustion of the Ca2+ store in irradiated mitochondria, the depolarizing effect of the uninterrupted neuronal membrane damage began to be dominant again, and acceleration phase reappeared. Firing acceleration went on until the irreversible destruction of spike-generating mechanism as in the case of the known cathodic or depolarization block process. The mechanism of cathodic block includes the inactivation of sodium channels and increase of potassium conductance leading to the elevation of spike generation threshold under prolonged depolarization [48]. The data on sodium channels inactivation in molluscan neurons under blue He-Cd-laser irradiation (441.6 nm) confirmed this assumption [15]. The irreversible neuron death mechanism in this case was probably necrotic because of the cell and nucleus swelling under the blue laser microirradiation [31] .
We suppose that the sequence of reactions described is common to different nonpigmented animal cells responding to a blue laser irradiation.
References
1. H.Senger (ed.) The Blue Light Syndrome, Springer-Verlag, Berlin, 1980.
2. H. Senger (ed.) Blue Light Effects in Biological Systems Springer-Verlag, Berlin, 1984.
3. T.I.Karu, Photobiology of Low-Power Laser Therapy, Harwood Academic, London, 1989.
4. T.I. Karu, Interaction of monochromatic visible light and near infrared radiation with cells: currently discussed mechanisms, Proc SPIE, 2391 (1995) 576-586.
5. A. Arvanitaki and N. Chalazonitis, Excitatory and inhibitory processes initiated by light and infra-red radiations in single identifiable nerve cells (giant ganglion cells of Aplysia), in Nervous Inhibition , Pergamon Press, Oxford et al. (1961) 194-231.
6. R.G. Lyudkovskaya and Yu. M.Burmistrov, Photobioelectric processes in excitable cells, in G.M. Frank (ed.) Biophysics of living cell , Puschino Press, Puschino (1971) 50-67.
7. A.M. Brown, P. S. Baur and F.H.Tuley, Phototransduction in Aplysia neurons: calcium release from pigmented granules is essential, Science,188, (1975) 157-160.
8. M.Henkart Light-induced changes in the structure of pigmented granules in Aplysia neurons, Science, 188 (1975) 155-157.
9. T.H. Nelson, J.I. Kim, and M. Kim, Photosensitivity of a bursting pacemaker neuron in Aplysia californica, Brain res.105 (1976) 583-587.
10. C.A. Erscine, Reaction of photosensitized glia cells and neurons in tissue culture to incondenscet light and electronic flash, Irish. Med. Sci., 395 (1958), 525-526.
11. R.L.Fork, Laser stimulation of nerve cells in Aplysia, Nature, 171 (1971) 907-908.
12. A.M. Prokhorov, F.V. Bunkin, G.A. Sitnikov, F.A. Logachev, K.V.Sudakov, S.A.Osipovsky, V.V.Savransky and A.P.Shurygina, Responses of edible snail central neurons to laser irradiation with different wavelengths Doklady AN SSSR, 251 (1980) 766-768 (in Russian).
13. A.B. Uzdensky, Laser microirradiation effect on isolated crayfish mechanoreceptor neuron, Biol.Sci.,No 3 (1980) 20-28 (in Russian).
14. T.A.Adzhimolaev, S.M.Zubkova and I.B.Laprun, Structural and functional changes in nerve cells at laser irradiation , in R.I.Utyamyshev (ed), Tools and Methods of Quantum Electronics in Medicine, Saratov Univ. Press, Saratov, (1976)156-159 (in Russian).
15. A.O. Korkushko, and E.L.Macheret, Mechanism of laser radiation action on neuron somatic membrane, Vrachebnoe Delo, No 7 (1982) 94-97 (in Russian).
16. I.E. Olson, D. Schimmerling, G.G.Gundy and C.A.Tobias, Laser microirradiation of cerebellar neurons in culture. Electrophysiological and morphological effects, Cell. Biophys., 3 (1981), 349-371.
17. P.Balaban, R.Esenaliev, T.Karu, E.Kukotina, V.Letokhov, A.Oraevsky, and N.Ovcharenko, He-Ne laser irradiation of single identified neurons, Lasers in Surg. and Med. 12 (1992) 329-337.
18. O.B., Il’insky, Physiology of sensory systems.Part III. Physiology of mechanoreceptors, Leningrad, Nauka. (1975) (in Russian).
19. E.E.,Giacobini, Chemical Studies of Individual Neurons , in Neurosciences Research. Part II. Invertebrate nerve cell, Acad.Press, New Jork, (1975) pp.111-202.
20. C.A.G.Wiersma, E. Furshpan and E.Florey, Physiological and pharmacological observations on muscle organ of the crayfish, Cambarus clarkii Girard, J.Exp.Biol., 30 (1953) 136-151.
21. S. Tschachotin, Die mikroskopische Strahlenstichmethode eine Zelloperationsmethode, Biol.Centralblatt, 32 (1912) 623-630.
22. M.W.Berns , Biological Microirradiation. Classical and Laser Sources, Engelwood Cliff., Prentice Hall, New York (1974)
23. M.W. Berns, N.Gamaleya, R.S. Olson et al., Argon laser micro-irradiation of mitochondria in rat myocardial cells in tissue culture, J.Cell.Physiol., 76 (1970) 207-214.
24. M.W. Berns, D.C. Gross, W.K.Cheng, D. Woodring, Argon laser micro-irradiation of mitochondria in rat myocardial cells in tissue culture. II. Correlation of morphology and function in single irradiated cells, J. Mol. Cell Cardiol. 4(1972) 71-83.
25. C. Salet , A study of beating frequency of a single myocardial cell. I. Q-switched laser microirradiation of mitochondria, Exp.Cell Res. 73(1972)360-366.
26. C. Salet, G.Moreno, F. Vinzens, A study of beating frequency of a single myocardial cell. II. Ultraviolet micro-irradiation of the nucleus and of the cytoplasm, Exp.Cell Res. 100 (1976)365-373.
27. Z.Lojda, Histochemistry of enzymes, Mir, Moscow (1982).
28. M.N. Kondrasheva, M.N. Lesogorova, and S.E. Shnol Method for assay of inorganic phosphates based on ultraviolet absorption spectra of molibdate complexes. Biochemistry 30 (1965) 567-571 (in Russian).
29. B.M. Vladimirsky,. Mathematical Methods in Biology, Rostov University Press, Rostov-on-Don (1983) (in Russian).
30. A.B. Uzdensky, The influence of laser microirradiation on isolated crayfish mechanoreceptor neuron, Biol.Sci., No 3 (1980) 20-28 (in Russian).
31. A.B. Uzdensky, Laser microirradiation of single nerve cell, in S.L. Jacques (ed.) Laser- Tissue Interaction IY. Proc.SPIE, 1882 (1993) 254-267.
32. A.B. Uzdensky, Laser microirradiation in investigation of integrative function of nerve cell, in Fercher et al. (eds) Microscopy, Holography, and Interferometry, Proc SPIE 2083 (1994) 225-234.
33. A.B. Uzdensky, Participation of some radical products in isolated nerve cell response to blue laser microirradiation, in G.Delacretaz et al. (eds) Laser Interaction with Hard and Soft Tissues. II., Proc. SPIE, 2323 (1994) 491-499.
34. A.B. Uzdensky, and V.V. Savransky, Single neuron response to pulse-periodic laser microirradiation. Action spectra and two-photon effect, J. Photochem. and Photobiol. B. Biology . 39 (1997) 224-228.
35. A. Lazarow,.and S.J. Cooperstein, Studies on the enzymatic basis for the Janus staining reaction, J.Histochem.and Cytochem. 1, (1953) 234-241.
36. G.M. Fedorenko, and A.B. Uzdensky, Isolated neuron ultrastructural changes induced by helium-cadmium laser micro- irradiation, Cytology 28, (1986) 512-516 (in Russian).
37. A.B. Uzdensky, Inactivation of succinate dehydrogenase in isolated crayfish mechanoreceptor neuron by focused blue laser radiation, Cytology, 29 (1987) 1392-1397 (in Russian).
38. B.B.Aggarwal, A.T. Quintanilha , R.Cammack, and L.Packer, Damage of mitochondrial electron transport and energy coupling byvisible light, Biochem. Biophys. acta, 502(1978) 367-382.
39. W. Schmidt, The study of basic photochemical and photophysical properties of membrane-bound flavins: the indispensible prerequisite for the elucidation of primary physiological blue light action. In H.Senger (ed.) Blue Light Effects in Biological Systems (1984) 81-94.
40. E.B.Burlakova, A.V. Alekseenko, et al. Bioantioxydants in radiation damage and malignant growth, Nauka, Moscow(1975) (In Russian).
41. N.V. Timofeev-Ressovsky, A.V.Savich, and M.I Shalnov, Introduction in Molecular Radiobiology, Medicina, Moscow (1981)(In Russian).
42. L.D. Luk'yanova, B.S. Balmuhanov, and A.T. Ugolev, Oxygen-dependent processes in cell and its functional state, Nauka, Moscow (1982)(In Russian).
43. E. Schonbohm, and E. Schonbohm, Multiple effects of the flavin quencher potassium iodide on light- and dark-processes in the green alga Mougeotia, In Senger H. (ed.) Blue light effects in biological systems, Springer-Verlag, Berlin, Heidelberg (1984) 137-145.
44. T. Pozzan, R Rizutto, P. Volpe, and J. Meldolesi, Molecular and cellular physiology of intracellular calcium stores. Physiol.Rew. 74 (1994) 595-636.
45. R.W.Meech, Intracellular calcium injection caused increased potassium conductance in Aplysia nerve cells," Comp.Biochem.Physiol. 42A (1972) 493-499.
46. R.W.Meech, Calcium influx induces a post-tetanic hyperpolarization in Aplysia neurones, Comp.Biochem.Physiol. 48A (1974) 387-395.
47. H. Low, and F.L. Crane, Redox function in plasma membrane Biochim.Biophys. Acta 515 (1978) 141-161.
48. B.I. Khodorov General Physiology of Excitable Membranes, Nauka, Moscow (1975) (In Russian).