

SUNLIGHT-PROMOTED PHOTOSENSITIZING AND PHOTOPHYSICAL PROPERTIES OF PORPHYRINS.
M. Tronchin ![]() , G. Jori
, G. Jori ![]() , M. Neumann
, M. Neumann ![]() , M. Schuetz
, M. Schuetz ![]() , A. Saiyadpour
, A. Saiyadpour ![]() and H.- D.
Brauer
and H.- D.
Brauer ![]()
| |
Corrispondence should be addressed to M. Tronchin
![]()
ABSTRACT
In order to start a "field" research line about potential porphyrin photopesticides six of them, namely Coproporphyrin (CP), Haematoporphyrin (HP), Protoporphyrin (PP), Zinc-Protoporphyrin (ZnPP), meso-Tetracarboxyphenylporphine (TCPP) and meso-Tetrasulphonatophenylporphine (TPPS) were chosen as representative of porphyrin main classes.
They were compared for theoretical sunlight-dependent singlet oxygen production effectiveness. For each molecule this parameter was calculated multiplying its singlet oxygen quantum yield by the overlap integral of the absorption spectrum with sunlight emission spectrum. With this parameter TCPP and PP seemed to be the more promising molecules. The overlap integral appeared to be the most important parameter to discriminate the theoretical sunlight-promoted efficiency. Only CP was limited by an other parameter: its singlet oxygen quantum yield.
In order to understand the influence of the more relevant photophysical properties,( i.e. triplet and fluorescence quantum yield, the energy of the first excited states, their lifetimes and quenching constants by molecular oxygen) of CP upon its singlet oxygen quantum yield we compared them with the ones of the other screened porphyrins.
![]()
INTRODUCTION
Porphyrins are among the first-used, the best-known photosensitizers. Their capability to accumulate in tumor cells and produce singlet oxygen during irradiation with visible light made these compounds extremely useful for the so-called photodynamic therapy of cancer (1) . New molecules are now conceived and experimented for improving tumor treatment (2) (3) and porphyrins, therefore, represent the "first generation" photosensitizers (3) to be used as a starting point. For the same reason other fields of application could take advantage of the experience accumulated upon porphyrins in tumor therapy. Light-activated pesticides is a developing area (4) where this experience might be employed. Of course a tumor is rather different from a fruit fly and conclusions should not be "tout coeur" used for testing.
The main difference between therapeutical and agricultural usage of photosensitizers is the light source. In therapy any light source may be theoretically chosen, (according e.g. the emission spectrum needed, power required...) and also the irradiation protocol fitted to the molecule to get best therapeutic needs (1). On the other hand the light source in field applications is sun only, and sensitizers must be chosen in order to fit it. During the day, moreover, sunlight could sometime be very weak and, as for any other kind of pesticide, forms of resistence like avoiding sunlight (4) may arise among pests.Even thought this kind of behaviuoral resistance turns into a self-limitation of pest diffusion, efficiency in killing parasites is always the primary target in order to limitate the pesticide doses. So absorption spectra, photosensitizing parameters, and correlated photophysical differencies between molecules, appear to be more critical in agricultural applications than in medical ones.
Sun-activated pesticides, and particularly porphyrins, on the other hand, show interesting positive properties with respect to traditional pesticides. Light may not penetrate deeply in human body (1) and therefore only sun-exposed areas of accidentally-poisoned persons are in danger. Few millimeters of light penetration is a limited portion of vertebrate body, while can be half of the body, or more, in insects. Sun-activated pesticides may therefore be selectively poisoning toward invertebrates. Thinking about a new class of pesticides one should consider also pollution. Among all photosensitizing molecules porphyrins are mostly naturally-occurring molecules, some of them normally present in human (and not only human) blood (Protoporphyrin), excretions (Coproporphyrin, Uroporphyrin) and therefore are good candidates for environmental-friendly photopesticides.
In order to start a photopesticide research line with porphyrins we screened six of them (see fig. 1), taken as representatives of their porphyrin classes, to determine the best candidate to sunlight-produced photosensitization. In order to understand the "bulk" efficiency results and allow future developments with different molecules we also determined their most important photophysical parameters.
![]()
MATERIALS AND METHODS
Porphyrins
(chemical structure in fig. 1) were obtained from Porphyrin Products Inc. and used without further purification. All other compouds were commercial spectroscopic-grade pure. In order to allow comparison we used ethanol or a 2% Triton-X100 micellar solution, unless differently specified, since they both proved to be good common solvents for all our porphyrins.Absorption spectra were obtained using a Perkin-Elmer Lambda-2 or a Lambda-5 spectrophotometer while fluorescence spectra were obtained with a Perkin-Elmer MPF4 or a 650-40 fluorimeter. Fluorescence quantum yields were determined using the steady-state comparative technique using acridine yellow (Qf = 0.47 in ethanol) as reference (15). All spectra were corrected for detector sensitivity.
Fluorescence lifetimes were determined by a home-built apparatus already described (16) using a Nitrogen laser (Lambda Physics). The emission wavelength (337 nm) was selected with interference and cutoff filters. The decay curves were analyzed with a computer program based on iterative deconvolution with least squares algorithm using a recursive formula (17). The fluorescence quenching by molecular oxygen was measured at three different oxygen concentrations in the range 1-3 mM.
Luminescence spectra of PP and ZnPP were obtained exciting with a mechanically-chopped light at 408 nm with a Xenon lamp as light source at 77°K in a glassy ethanol/water (99:1) mixture using a home-built instrument (18) with a monochromator connected to a nitrogen-cooled Germanium diode (North Coast Scientific Corp. EO) and whose signal was filtered by a muon filter before passing to a lock-in amplifier connected to a computer for data digitalization. Shifting the phase delay allowed us to read luminescence in the phosphorescence contribution-only area while reading total luminescence at room temperature gave the fluorescence-only light contribution. These fluorescence spectra were compared with the ones obtained with fluorimeters and used as a control. All spectra were corrected for detector sensitivity. For the determination of the energy of the T1 states of the other porphyrins, according to the procedure of Dreeshamp et al (19) (20), the fluorescence quenching by iodopropane was measured in degassed ethanol at five concentration in the range (0.2 - 1) x 10^-6 M.
Singlet oxygen was determined using the the steady-state 1270-nm luminescence comparative techniques, with perinaphtenone (Qdelta = 0.97) as reference (21) and the same Germanium-diode apparatus as described above. To improve the signal the monochromator was substitued by an interference filter.
Front-face photoacoustic calorimetry (22) in DMSO provided the data for evaluating the triplet quantum yields (Qisc in the absence of oxygen) of PP and ZnPP.
The apparatus for determination of true lifetime of triplet states and their interaction with oxygen was decribed in detail elsewhere (23). Excitation source (lambda = 308 nm) was a Xell excimer laser (Lambda Physics). The oxygen quenching of the T1 states was measured at three oxygen concentrations in the range 0.1- 0.3 mM.
![]()
RESULTS AND DISCUSSION
Absorption spectra
in 2% triton micelles were measured for all compounds. EPSILON(max) of the Soret band (tab 1) was obtained from literature or calculated according to Lambert-Beer law.The molar extinction coefficient (EPSILON) is often considered as an important parameter for evaluating the theoretial effectiveness of a photosensitizer. From this point of view meso-substituted porphyrins show the highest values, over 3 x 10^5 M^-1 cm^-1 whereas ZnPP the lowest (above 1). Actually the molar extinction coefficient at one single wavelength is just a rough parameter for evaluating absorption efficiency. It is necessary to take into account the whole absorption spectrum and the emission spectrum of the light source. Then EPSILON(max) and absorbance data A(lambda) form the spectra were used to calculate EPSILON(lambda) for each molecule using the formula
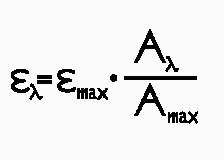 (1)
(1)
Text version of formula (1): EPSILON(lambda) = EPSILON(max) * A(lambda) / A(max)
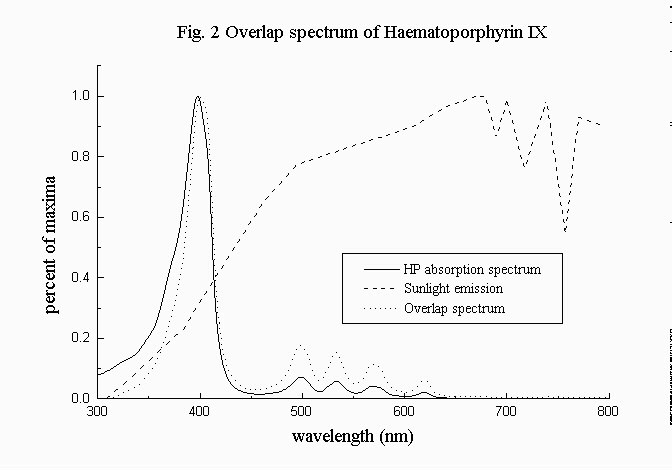
These EPSILON(lambda) values were used for calculating the overlap integral Psigma (fig 2) with the sunlight emission (10) using the formula.
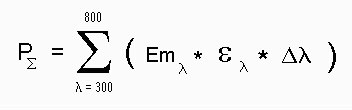 (2)
(2)
Text version of formula (2): Psigma = Sum of ( EPSILON(lambda) * SUNLIGHT-EMISSION(lambda) * delta(lambda)) with lambda 300 nm -> 800 nm
If differencies in EPSILON(max) were striking (the EPSILON(max) values vary from 0.9 to 3.5 (x 10^5 M^-1 cm^-1), i. e. the highest value is roughly fourfold the lowest), the overlap integral values show more homogeneous values.
Besides ZnPP, (that still keeps the lowest Psigma value) (< 0.5 x 10^21 E M^-1 s^-1 cm^-3) calculated absorption efficiencies (tab 1) ranges from 0.8 to 1.7 (x 10^21 E M^-1 s^-1 cm^-3) i. e. the highest value is twofold the lowest one. TCPP has still the highest value but the value of TPPS is lower than those of CP and PP,and equals that of HP.
These values, however, can provide only "how efficiently" our compounds can absorb sunlight but, by themselves, give no information about the photosensitizing efficiency differencies.
In order to evaluate the theoretical photosensitizing efficiency of a molecule we must take into consideration also the singlet oxygen quantum yield (Qdelta). So we multiplied these integrals for the singlet oxygen quantum yield according to the formula.
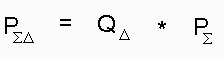 (3)
(3)
Text version of formula (3) PSigma-Delta = Qdelta * Psigma
In this way we obtained what we called "Sunphotosensitizing efficiency parameterr" (PSigma-Delta). Results are shown in table 2.
Besides CP, singlet oxygen quantum yields are mostly similar (0.8 - 0.9). ZnPP shows the highest value, but using as parameter PSigma-Delta we must conclude that TCPP and PP are the best sunlight-promoted sensitizers among the selected ones, In our group of porphyrins the similarity of Qdelta values makes the overlap integral Psigma the most important parameter. Only CP has a Qdelta value which is strongly limitating its efficiency.
For a more complete understanding of the photosensitizing efficiency properties and potentialities of our compounds we determined also the "Triplet formation efficiency parameter" (PSigma-T) with a formula very similar to the one used for calculating PSigma-Delta i. e.
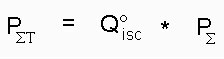 (4)
(4)
Text version of formula (4): PSigma-T = Q°isc * Psigma
Results are shown in table 3. The values of PSigma-T confirm mostly the PSigma-Delta values in table 2 with one exception, namely CP. Only for this porphyrin a distinct difference is observed between the Qdelta-value and the Q°isc-value, indicating that the oxygen quenching of the T1-state of CP does not only occur via an energy transfer process generating singlet oxygen (vide infra).
To get more insight with respect to the singlet oxygen
generation we have determined the energies of the S1 and T1
states of the porphyrins.
Furthermore we have measured the
lifetimes of the S1 states of the porphyrins as a function of the
oxygen concentration. Additionally for PP the rate constant of
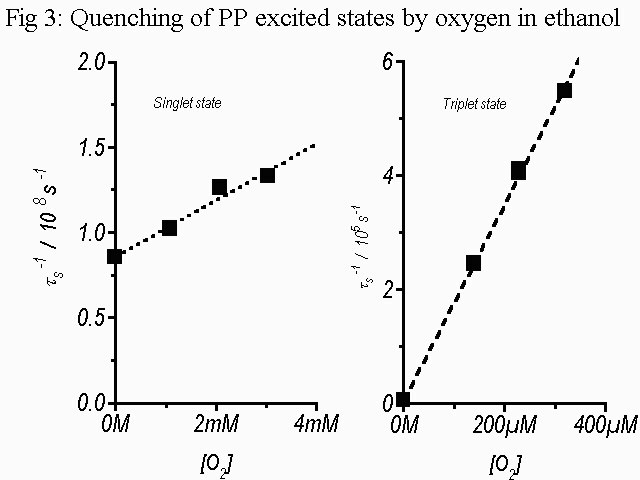
the oxygen quenching of the T1 state was determined in ethanol. Fig. 3 shows the Stern-Volmer plots of
the oxygen quenching of the lowest excited states of PP in
ethanol from which the quenching constants Kq(S) and Kq(T)
respectively were evaluated.
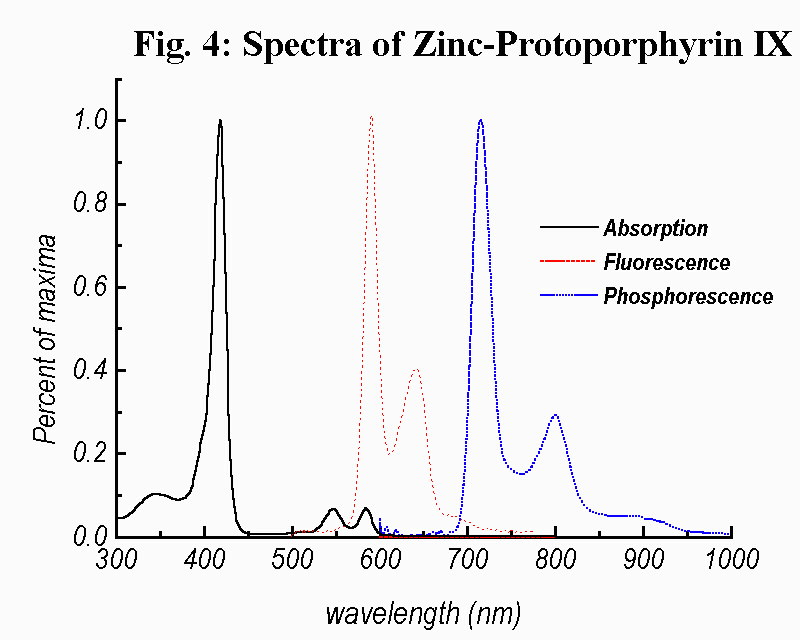
Fig. 4. exibiths, besides the absorption spectrum recorded in ethanol, the emission spectra of ZnPP both recorded in an ethanol/water (99:1) mixture at different temperatures. From the phosphorescence spectrum the energy of the T1 state was determined.
In table 4 the energetic and kinetic data of the S1 states of porphyrins are summarized. Table 5 shows the corresponding data of the T1 state determined by ourselves and other authors, respectively.
In accordance with the findings of other porphyrin derivatives also for the porphyrins investigated the S1-T1 energy gap is distinctively smaller than the singlet oxygen (1-delta-g) excitation energy of about 94 kJ mol^-1. Conseguentely singlet oxygen can only be produced by oxygen quenching of the T1 state of the porphyrins.In this case Qdelta can be expressed by the formula (eq. 5.)
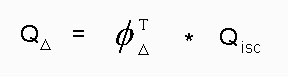 (5)
(5)
Text version of formula (5): Qdelta = Phi(delta-T) * Qisc
where Phi(delta-T) is the fraction of singlet oxygen formation accompaning the oxygen quenching of the T1 state of the porphyrins and Qisc denotes the porphyrin quantum yields of the S1 -> T1 intersystem crossing in the presence of oxygen.
For Qisc the following formula (eq. 6) holds:
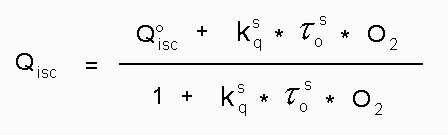 (6)
(6)
Text version of formula (6): Qisc = ( Q°isc + Kq(S) * Tau°(S) * [Oxygen] ) / ( 1 + Kq(S) * Tau°(S) * [Oxygen] )
with the values of Q°isc, Kq(S) and Tau°(S) presented in table 3 and table 4, respectively, and the oxygen concentration of about 2.1 x 10^-3 M valid for air-saturated ethanol (24) values of Qisc can be calculated which are only a little bit greater (2-7%) than the Q°isc values.
The values of Phi(delta-T) calculated with eq. (5) are also given in table 5. On the basis of these values only for CP can be concluded that the T1 state quenching by molecular oxygen does not occur exclusively via an energy transfer process.
However, in ethanol for both PP and CP Kq(T) values have found to be smaller than 1/9 Kdiff = 2.8 x 10^9 M^-1 s^-1 (Kdiff(ethanol) = 2.5 x 10^10 M^-1 s^-1) (24). This results is considered with the assumption that the triplet quenching by molecular oxygen of both porphyrins follows only via the energy transfer process (I) and that the process (II) does not take place.
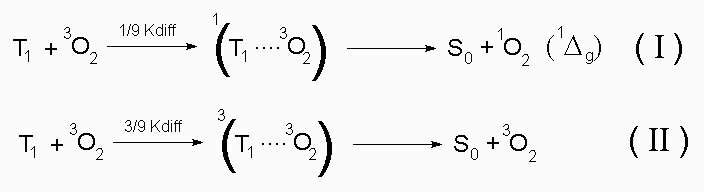
Therefore at present it is not possible to make a sure statement about the oxygen quenching mechanism of the T1 state of CP.
![]()
CONCLUSIONS
Among selected porphyrins TCPP is the most efficient one in generating sunlight-promoted singlet oxygen. Its four carboxyl groups make the molecule well soluble in water and therefore easy to handle for field applications against pests. On the other hand water-soluble molecules are known to be rapidly excreted. Experiments by Bruni and Ben Amor (personal comunication) with fruit flyes show no pest death with TCPP, while HP, with half the PSigma-Delta of TCPP, shows a very good killing efficiency.
From a structural point of view PP is very similar to HP, and on the basis of our data, it could be a very good alternative to HP because of its higher PSigma-Delta.
Among similar porphyrins the overlap spectrum (Psigma) seems to be the most important factor in influencing PSigma-Delta. EPSILON and Qdelta may play a major role only in some cases (CP). In any case many elements must be taken into consideration since the factors that influence sunlight-promoted sensitization efficiency (PSigma-Delta) are numberous and interact in a complex way.
![]()
ACKNOWLEDGMENT
Financial support by the funding of the Human Capital and Mobility programme of the EU (PDT Euronet CHRX-CT 93-0178) is gratefully acknowledged.
![]()
Table 1
Absorption properties of selected porphyrins |
||
| Porphyrin | EPSILONmax * | Psigma ** |
| Coproporphyrin (CP) | 2.3 | 1.4 |
| Haematoporphyrin (HP) | 1.3 | 0.8 |
| Protoporphyrin (PP) | 1.6 | 1.6 |
| Zinc-Protoporphyrin (ZnPP) | 0.94 (13) | 0.48 |
| meso-Tetrasulphonatophenylporphine (TPPS) | 3.5 | 0.8 |
| meso-Tetracarboxyphenylporphine (TCPP) | 3.3 | 1.7 |
- Legend: values of EPSILONmax (Soret band) and Psigma of the porphyrins valid for 2% triton micelles
- * EPSILONmax is expressed as 10^5 M^-1 cm^-1
** Psigma is expressed as 10^21 Einstein M^-1 s^-1 cm^-3
(13) denotes the literature value
![]()
Table 2
Singlet oxygen formation of selected porphyrins |
||
| Porphyrin | Qdelta * | PSigma-Delta ** |
| Coproporphyrin (CP) | 0.58 (11) | 0.8 |
| Haematoporphyrin (HP) | 0.84 | 0.7 |
| Protoporphyrin (PP) | 0.77 | 1.2 |
| Zinc-Protoporphyrin (ZnPP) | 0.90 | 0.43 |
| meso-Tetrasulphonatophenylporphine (TPPS) | 0.80 (11) | 0.6 |
| meso-Tetracarboxyphenylporphine (TCPP) | 0.83 | 1.4 |
- Legend: values of singlet oxygen quantum yield (Qdelta) and Sunphotosensitizing efficiency parameter (PSigma-Delta)of the porphyrins.
- * Qdelta was determined in ethanol
** PSigma-Delta is expressed as 10^21 Einstein M^-1 s^-1 cm^-3
(11) indicates the literature value
![]()
Table 3
Triplet formation of selected porphyrins |
||
| Porphyrin | Q°isc * | PSigma-T ** |
| Coproporphyrin (CP) | 0.81 (11) 0.88 (5) |
1.2 |
| Haematoporphyrin (HP) | 0.92 (5) 0.94 (6) |
0.8 |
| Protoporphyrin (PP) | 0.80 (5) 0.68 *** |
1.3 |
| Zinc-Protoporphyrin (ZnPP) | 0.90 (13) 0.77 *** |
0.43 |
| meso-Tetrasulphonatophenylporphine (TPPS) | 0.76 (7) | 0.6 |
| meso-Tetracarboxyphenylporphine (TCPP) | 0.85 (11) | 1.5 |
- Legend: values of intersystem-crossing quantum without oxygen (Q°isc) and Triplet formation efficiency parameter PSigma-T of the porphyrins.
- * Q°isc was determined in ethanol (besides values with
***)
** PSigma-T is expressed as 10^21 Einstein M^-1 s^-1 cm^-3
*** values measured in DMSO, not used for calculating PSigma-T
the numbers in the brackets indicates the literature value
![]()
Table 4
Photophysical data about the first singlet state |
||||
| Porphyrin | Qf * | Energy ** | Tau°(S) *** | Kq(S)(O2) **** |
| Coproporphyrin (CP) | 0.01 | 193 | 19 | 12.4 |
| Haematoporphyrin (HP) | 0.03 | 193 | 18 | 11.9 |
| Protoporphyrin (PP) | 0.05 | 190 | 11 | 15.2 |
| Zinc-Protoporphyrin (ZnPP) | 0.04 | 197 | 1.8 (13) | -- |
| meso-Tetrasulphonatophenylporphine (TPPS) | 0.02 | 185 | 13 | 10.9 |
| meso-Tetracarboxyphenylporphine (TCPP) | 0.03 | 183 | 12 | 9.5 |
- Legend: Photophysical data of the excited state of the porphyrins determinated in ethanol.
- * Fluorescence quantum yield in air-saturated ethanol
** Energy of the state is expressed as kJ/mol
*** Tau°(S) denotes the fluorescence lifetime in the absence of oxygen, it is expressed as ns
**** Kq(S)(O2) denotes the quenching constant of the excited state by oxygen, it is expressed as 10^9 M^-1 s^-1
(13) denotes the literature value
![]()
Table 5
Photophysical data about the first triplet state |
||||
| Porphyrin | Energy * | Tau°(T) ** | Kq(T)(O2) *** | PHI(delta-T) * |
| Coproporphyrin (CP) | 142 | 1.8 (9) | 1.8 | 0.67 |
| Haematoporphyrin (HP) | 159 (8) | 0.8 (9) | 1.4 (5) 2.0 (9) |
0.88 |
| Protoporphyrin (PP) | 171 | 0.18 | 1.9 2.7 (5) |
0.91 |
| Zinc-Protoporphyrin (ZnPP) | 167 | 0.22 | 2.9 (13) | 1.00 |
| meso-Tetrasulphonatophenylporphine (TPPS) | 137 | 1.4 | 1.9 (5) | 0.99 |
| meso-Tetracarboxyphenylporphine (TCPP) | 149 | 0.98 | 1.9 (5) | 0.98 |
- Legend: Photophysical data of the excited state of the porphyrins determinated in ethanol.
- * Energy of the state is expressed as kJ/mol
** Tau°(T) denotes the triplet lifetime in the absence of oxygen, it is expressed as ms
*** Kq(T)(O2) denotes the quenching constant of the excited state by oxygen, it is expressed as 10^9 M^-1 s^-1
(13) denotes the literature value
****PHI(delta-T) denotes the efficiency of singlet oxygen formation from triplet state
![]()
Figure 1
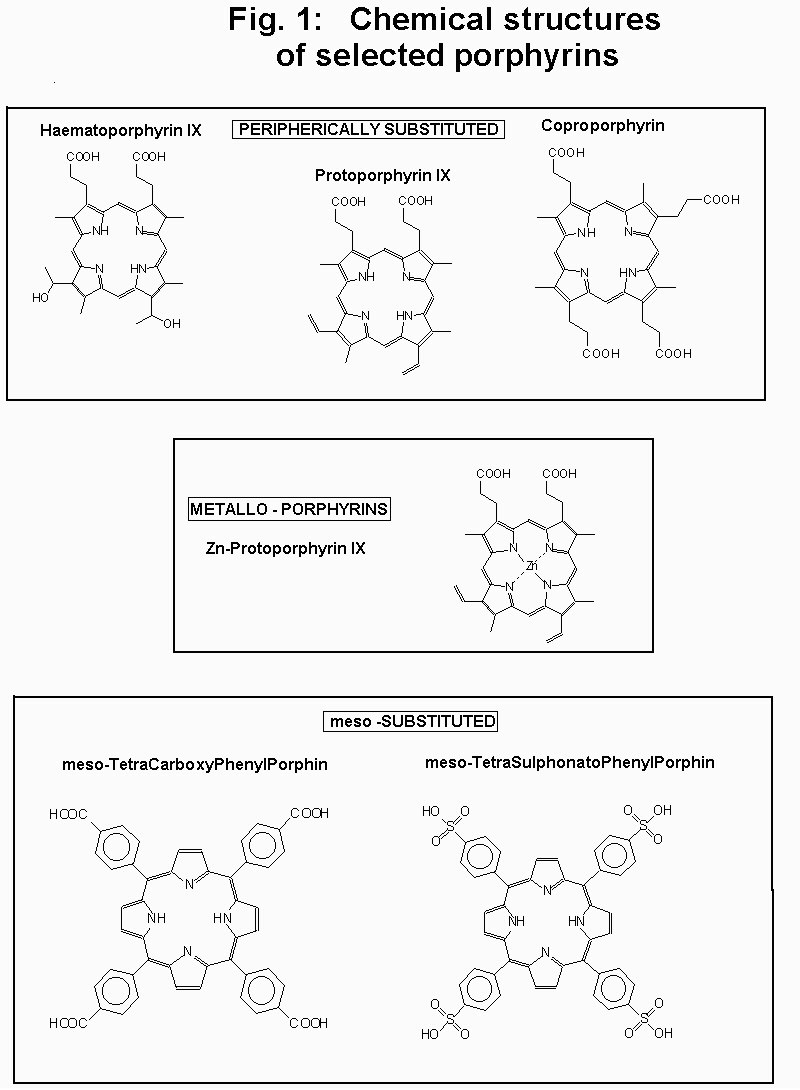
![]()
Figure 2
- Back to quotation 1
![]()
Figure 3
Stern-Volmer plots 1/Tau versus [O2] for the oxygen quenching of the S1 and T1 states of PP in Ethanol.
- Back to quotation 1
![]()
Figure 4
Absorption Spectrum (in ethanol) and luminescence Spectra (in ethanol/water (99:1) mixture) of Zn-Protoporphyrin
- Back to quotation 1
![]()
BIBLIOGRAPHY
- Spikes J., Jori G., "Photodynamic Therapy of Tumors and other Diseases using Porphyrins" Lasers in Medical Sciences, 3 (1987) 3.
- Schaffner K., Vogel E., Jori G. "Porphycenes as Photodynamic Therapy Agents" in "Biological Effects of Light" ed Walter de Gruyer & Co., (1993) 312.
- Kreimer-Birnbaum "Modified porphyrins, chlorins, phthalocyanines and purpurins: second generation sensitizers for photodynamic therapy" Semin. Hematol., 26 (1989) 157.
- Berenbaum M. R. "Charge of the Light Brigade: Phototoxicity as a Defence against Insects" in "Light-activated Pesticides", ACS Symposium serie, 339 (1987) 206.
- Bonnet R, Lambert C., Land J., Scourides P. A., Sinclair R. S.: " The triplet and radical species of Haematoporphyrin and some of its derivates" Photochem. Photobiol., 38 (1983) 1.
- Keene J. P., Kessel D., Land E. J., Redmond R. W., Truscott T. G. "Direct detection of singlet oxygen sensitized by Haematoporphyrin and related compounds" Photochem Photobiol., 43, (1986) 117
- Bonnet R., Ridge R. J., Land E. J., Sinclair R. S., Tait D., Truscott T. G. "Pulsed irradiation of water-soluble porphyrins" J. Chem. Soc., Faraday Trans., 78 (1982) 127.
- Reddi E., Valduga G., Rodgers M. A. J., Jori G. "Studies on the mechanism of the Hematoporphyrin-sensitized photooxydation of 1,3-diphenylisobenzofuran in ethanol and unilamellar liposomes" Photochem. Photobiol., 54 (1991) 633
- Reddi E., Jori G., Rodgers M. A. J. "Flash Photolysis studies of Hemato- and Copro- porphyrins in homogeneous and microheterogeneous acqueous dispersions" Studia Biophysica, 94 (1983) 13.
- Selinger in "The Science of Photobiology " by K. C. Smith ed. Plenum Publishing Corp., (1977) 145.
- Lambert, Reddi, Spikes, Rodgers, Jori "The effect of porphyrin structure and aggregation state on photosensitized proceses in acqueous and micellar media" Photochem. Photobiol., 44 (1986) 595.
- Rimington "Spectral absorption coefficients of some porphyrins in the Soret-band region" Biochem. J., 75 (1960) 620.
- Feitelson J., Barboy N.: "Triplet-state reactions of Zinc-Protoporphyrins" J. Phys Chem., 90 (1986) 271.
- Heitz J. R. "Development of Photoactivated compunds as Pesticides" in "Light-activated Pesticides", ACS Symposium serie, 339 (1987) 1.
- Olmsted J. "Calorimetric determination of absolute fluorescence" J. Phys. Chem., 83 (1979) 2581.
- Grewer Ch., Brauer H.-D.
"Temperature dependence of the oxygen quenching
 -singlet and
-singlet and  -triplet
states of singlet oxygen sensitizers", J. Phys
Chem., 97 (1993) 5001.
-triplet
states of singlet oxygen sensitizers", J. Phys
Chem., 97 (1993) 5001. - Grewer Ch., Wirp Ch., Neumann M., Brauer H.D., "Evidence for competing electron and energy transfer for the fluorescence quenching of singlet oxygen sensitizers by molecular oxygen" Ber. Bunsenges. Phys. Chem., 98 (1994) 997.
- A. Völcher, H.-J. Adich, R. Schmidt, H.-D. Brauer "Near infra-red Phosphorescence emission of compounds with low-lying triplet states" Chemical Physics Letters, 159 (1989) 103.
- Dreshamp H., Koch E., Zander M., "Schweratom-induzierte Fluoreszenzlöschung und Termschema von mehrhernigen aromatischen Kohlenwasserstoffen und Heterocyclen". Ber. Bunsenges. Phys. Chem., 78 (1974) 1328.
- Wirp Ch., Bendig J., Brauer H.-D., "Singlet and triplet quenching of aromatic cations by molecular oxygen". Ber. Bunsenges. Phys. Chem. , 101 (1997) 961.
- R. Schmidt, C. Tanielan, R. Dunsbach, C. Wolff "Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2 (1Deltag) sensitization" J. Photochem. Photobiol. A: Chem., 79 (1994) 11.
- Dunsbach R., Schmidt R., "Photophysical properties of some polycyclic conjugated hydrocarbons containing five-membered rings" J. Photochem. photobiol. A: Chem., 83 (1994) 7.
- Scherman G., Völker A., Seikel K., Schmidt R., Brauer H.-D., Horstforts F.-P., "Potential photosensitizers for Photodynamic therapy - I. Photophysical properties of two chlorin-derivatives". Photochem. Photobiol., 51 (1990) 45.
- Ware w. R. "Oxygen quenching of fluorescence in solution: an experimental study of the diffusion process" J. Phys. Chem., 66 (1962) 455.