Assessment of Euglena gracilis as a biological dosimeter for solar UVA and UVB under field conditions
BY
Katerina Koussoulaki1, Daniel Danielidis2, D. –P. Häder3 and Regas Santas1*
1 OikoTechnics Institute, Kefallenias 50, Athens GR-16342
2 University of Athens, Division of Ecology and Systematics, GR-15784
3 Friedrich-Alexander Universität, Staudtstr. 5, Erlangen, D-8520
* corresponding author
KEY WORDS
Ozone depletion, flagellates, biological dosimetry, solar UVR, image analysis, Greece.
ABSTRACT
The potential of using Euglena gracilis in biological dosimetry was assessed by exposing pure cultures to filtered and unfiltered solar radiation for 3, 7 and 14 days. Cell motility and velocity were determined by a real time image analysis system. After 3 days the reduction in mean motility of E. gracilis was more dramatic under the PAR+UVA+UVB treatment, while in the PAR+UVA treatment it was intermediate (61.3%) between the PAR+UVA+UVB (37.1%) and the PAR treatments (78.4%). Cell motility was highest (88.2%) in the dark. Motility in the PAR+UVA treatment was significantly lower than in the control and PAR treatment. On day 7, motility in the PAR+UVA+UVB treatment (24.3%) was significantly lower than all other treatments. After 14 days of exposure motile cells were found in the control cultures but not in any light treatment. Evidence was found that cell mean speed tends to be higher in the presence of UVR. These assays suggest that E. gracilis is a suitable biological dosimeter for field assays of 3-10 days of full sunshine in the Mediterranean region.
INTRODUCTION
The rapid decline in stratospheric ozone concentrations1 and the associated increase in ambient UVB has been shown to have deleterious effects on living organisms. The need to obtain a measure of the biological effects of solar UV within a relatively short time period has prompted several biological dosimetry investigations. Biological dosimetry involves the exposure of a sensitive organism to UV, and the subsequent assessment of the biological risk posed based on relevant morphological, biochemical and physiological observations. Bacteriophage T7,T4,T1 and f X174 been used in such experiments as UV biosensors.2-6 Other organisms proposed as UV biosensors include the bacteria Escherichia coli K12 and Bacillus subtilis, 6-11 as well as cyanobacteria.12-14 These investigations have shown a negative correlation between organism survival and UVR.
However, it is questionable whether UV-inflicted damage on dark-adapted organisms can be extrapolated for predicting biological effects at the ecosystem level. Unlike viruses and most bacteria, photosynthetic organisms depend directly on light availability for their survival and thus have fewer alternatives for niche selection. Therefore, the use of photosynthetic microorganisms may offer a more realistic biological dosimetry approach. Enhanced levels of UVB radiation have harmful effects on the survival, motility and/or orientation of aquatic photosynthetic microorganisms such as cyanobacteria, red, green and brown algae.15-17
Euglena gracilis, a photosynthetic freshwater flagellate, has been extensively investigated for its behavior under exposure to artificial UVB radiation and simulated solar radiation. UVB was found to alter cell shape18 and chloroplast development.19,20 Exposure to UVB also affects several biosynthetic pathways connected with the chloroplast,19,20 and other metabolic processes such as calcium uptake.21 In addition, UVB affects cell motility by impairing the normal function of the flagellum.22-24 Cell motility effects include the loss of gravitactic orientation ability and interference with phototactic rhythms.18,23,25-27
Most of the above studies were conducted under artificial UVB levels several times higher than the natural incidence of solar UVB irradiance. This study was undertaken as a first attempt to evaluate the potential of using E. gracilis as a biological dosimeter for field assays. To meet this goal, the effects of PAR, UVA and UVB were assessed on cell motility by exposure to natural sunlight in the field and artificial UVB in the laboratory.
METHODS AND MATERIALS
Organisms and culture conditions
Euglena gracilis was cultured in a mineral medium consisting of CH3COONa.3H2O, (NH4)2HPO4, KH2PO4, MgSO4.7H2O, CaCl2.2H2O, EDTA-II, FeCl3, vitamins B1, B12 and trace elements. Cells were grown in static cultures at 20oC.
Exposure to artificial radiation
E. gracilis cultures were irradiated with monochromatic UVB radiation in the laboratory using a Bachofer, Reutlingen, FRG transilluminator at 23oC. The spectral output of this source closely resembles that of the Phillips TL 12 lamps filtered by an I1-200-M filter for removal of the UVC component. The transilluminator emits UVB radiation with a peak output at 312 nm. Organisms were exposed to two levels of UVB irradiance: 15.9 and 2.4 W/m2 using neutral density filters to reduce the output of the transilluminator. The percentage of motile cells and the mean value of their speed and orientation were measured every five minutes for a total of 75 minutes.
Exposure to solar radiation
Outdoor experiments were conducted in Ancient Korinthos, Greece (37o 58’ N, 23o 0’ E). E. gracilis cultures were exposed to solar radiation during the period of May - July 1996 in Plexiglas chambers placed at a depth of 10 cm under the water surface. Attenuation of the PAR (400-800 nm), UV-A (315-395 nm) and UV-B (290-315 nm) bands in the water column was measured with an Optronic 752 double monochromator spectroradiometer. Water temperature ranged from 18-25°C during the night and 25-30°C in the course of the day.
Using UV-transmitting Plexiglas (GS 2458) and plastic foil cutoff filters, (295 Ultraphan; PR Montagefolie 320 nm Art. Nr. 10155 099; and 395 Ultraphan UV Opak; thickness: 0.3 mm), 3 treatments were performed as shown in Table 1. The percentage of motile cells, their mean speed and the total number of individuals per optical field were measured every 3, 7 and 14 days. In all experiments motility parameters were also measured in replicate cultures kept in the dark, at the same depth and temperature.
Table 1: Light treatments
| Treatment | PAR+UVA+UVB |
PAR+UVA |
PAR |
Dark |
| cutoff (nm) | 295 |
320 |
395 |
- |
Cell counts
A real time image analysis program28,29 was used to measure cell number, motility and mean speed. The system consists of a microscope (Nikon 104) with a 6.3x objective lens, a video camera (CCD Philips LHD 0600) adapted on the microscope. The video signal was digitized in real time (Matrox PIP 1024, Quebec, Canada) with a spatial resolution 512 x 512 pixels at 256 possible gray levels. A PC computer analyzed the image data using a software package written in C and Assembly computer languages.30 The software recognizes the cells as groups of dark pixels. Real time monitoring of cell motility is achieved through consecutive "snapshots" taken at fractions of a second. The velocity of the organisms was calculated from the distance they had moved in the time determined from the computer clock. Since no statistical differences were observed in the cell motility and velocity patterns for the same treatments between the three runs, the pooled data were subjected to one-way ANOVAs; Tuckey's test was used for means analysis. 95% Just Significant Confidence Intervals (JSCIs) were calculated and placed around the means.31 Overlapping of JSCIs indicate not significantly different means.
RESULTS AND DISCUSSION
Exposure to artificial UVB
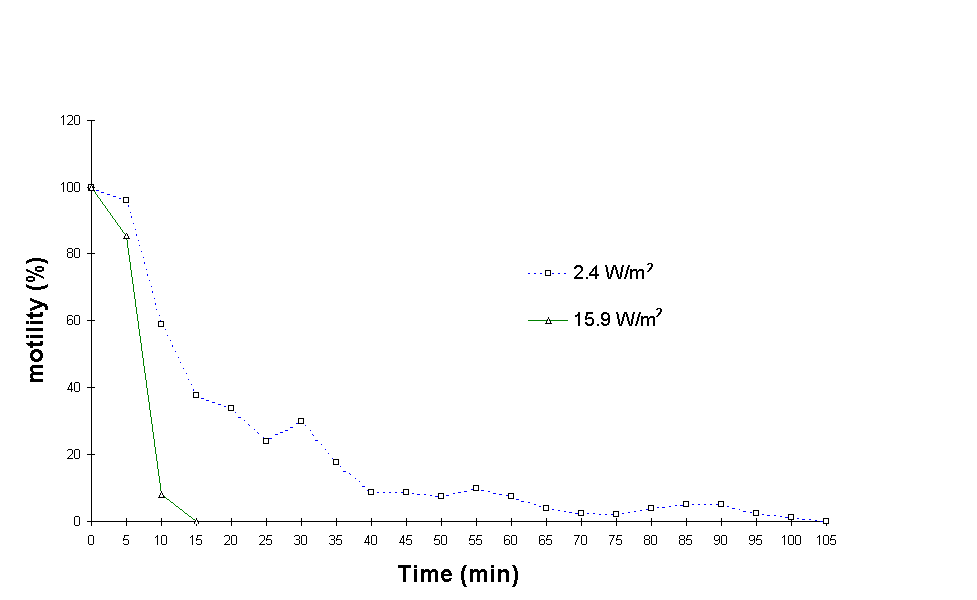
Figure 1: Cell motility during exposure to artificial UVB.
When exposed to 15.9 W/m2 of UVB radiation in the laboratory, the cell motility of E. gracilis dropped to 8% after 10 minutes and reached 0% within the next 5 minutes, at a total UVB dose of 14,310 J m-2 (Figure 1). At 2.4 W m-2, cell motility was reduced to 37.5% within 15 minutes (total dose 2,160 J m-2). Cell motility dropped to 9% after 40 minutes of exposure (total dose 5,760 J m-2) and gradually reached 0% after 105 minutes of irradiance (total dose 15,120 J m-2). It is worthwile noticing that the total doses required to immobilize the cultures are approximately equal under the two irradiances.
At 2.4 W m-2, the reduction in cell mean speed was more gradual reaching 0 m m/s after 105 min of exposure (Figure 2). Under 15.9 W m-2, cell mean speed dropped to 0 m m/s after the first 15 minutes.
Figure 2: Cell mean speed during exposure to artificial UVB.
Exposure to solar radiation
The underwater spectrum under the 3 light treatments is given in figure 3.
Figure 3: Underwater solar spectrum in the three light treatments.
The maximum irradiance of solar UVB as measured by an ELDONET dosimeter32 was 0.76 W m-2 during mid-day in May, 0.97 W m-2 in June and 1.06 W m-2 in July. The total doses of the three bands during the exposure intervals of the three runs are presented in Table 2. Total UVB doses ranged between 38 kW m-2 for the 3 day exposure (May) to 301 kW m-2 for the 14 day exposure (June), while the UVA and PAR doses ranged between 2,586 and 26,205 kW m-2 (3 days, May) and 15,891 and 150,742 kW m-2 (14 days, June), respectively.
Table 2. Total solar UVB, UVA and PAR radiation doses (kW m-2)
UVB |
UVA |
PAR |
|||||||
days month |
3 |
7 |
14 |
3 |
7 |
14 |
3 |
7 |
14 |
| MAY | 38 |
89 |
168 |
2,586 |
6,134 |
12,568 |
26,205 |
62,146 |
124,296 |
| JUNE | 63 |
144 |
301 |
3,234 |
7,506 |
15,891 |
32,946 |
76,074 |
150,742 |
| JULY | 58 |
139 |
260 |
3,185 |
7,492 |
14,663 |
30,004 |
71,099 |
142,067 |
| average | 53 |
124 |
243 |
3,002 |
7,044 |
14,374 |
29,718 |
69,773 |
139,035 |
Figure 4 depicts percent motility of the cultures averaged over the three experiments during the 14-day exposure intervals. Cell motility in the PAR+UVA+UVB treatment was significantly lower than all other treatments on days 3 and 7 (figure 5). After 3 days of exposure the reduction in mean motility of E. gracilis was more dramatic under the PAR+UVA+UVB treatment (37.1%), while in the PAR+UVA treatment cell motility (61.3%) was intermediate between the PAR+UVA+UVB and the PAR treatments (78.4%). Cell motility was highest (88.2%) in the dark. Motility in the PAR+UVA treatment was significantly lower than in the control and PAR treatment.
Figure 4: Cell motility during exposure of E. gracilis to solar radiation.
Figure 5: Mean cell motility of the 3 runs under the 3 light treatments and control. Bars are 95% just significant confidence intervals (F=33.649; df=7, 29; p<0.05).
On day 7, motility in the PAR+UVA+UVB treatment (24.3%) was significantly lower than all other treatments except the corresponding value for day 3. Motility in PAR+UVA treatment and the control also decreased (70.2 and 75.2% respectively), but increased in the PAR treatment (83.1%; figures 4, 5), possibly because of vegetative growth. After 14 days of exposure no motile cells were detected in any light treatment, probably because of starvation due to nutrient depletion in the experimental chambers. Motility in the dark, however, increased to 84.6%. This probably marks the switching of organisms from autotrophic to heterotrophic habit. The slight decrease in cell motility from day 0 to day 7 (figure 4) in the control can be explained by the depletion of storage compounds before the switching of trophic status. On day 14, some motility might have been expected under some of the light treatments. However, the zero values observed in all light treatments including PAR, suggest that the presence of light inhibits the switching of E. gracilis to a heterotrophic state which would in turn have enabled the organisms to survive.
Cell mean speed was highest in the PAR+UVA treatment on days 3 and 7. However, the only statistically significant difference observed was that the PAR+UVA treatment was higher than the control and PAR+UVA+UVB treatments on day 7. Cell mean speed in the PAR+UVA+UVB treatment was higher, although not significantly different than that of the control and PAR treatment on day 3. These results suggest that cell mean speed tends to be higher in the presence of UVR. However, this could not be documented in the present study, as the organisms were restricted in a closed experimental chamber.
Figure 6: Mean cell velocity of the 3 runs under the 3 light treatments and control. Bars are 95% just significant confidence intervals(F=3.581; df=7, 29; p<0.05).
In the laboratory, relatively small total UVB doses (c. 15 kW m-2) produced by short exposure to high irradiances of artificial UVB resulted in zero motility and velocity values. By contrast, in the field, the cultures showed vital signs of mobility and velocity on day 7, when the total UVB dose was more than 8 times the laboratory value (124 kW m-2). This phenomenon can be explained by a) repair mechanisms - both photorepair by UVA and PAR and dark repair, and b) the smaller UVB incidence in the field (c. 1 W m-2 in the field vs. 2.4 and 15.9 W m-2 in the laboratory). Other researchers have pointed out that the dose does not always cause an equal effect for a short exposure to a high intensity source as a long exposure to a low intensity source even though the products are equal.33 Visible light is known to be remedial to chloroplast formation irregularities in E. gracilis, induced by ultraviolet radiation.20 With regard to dark repair in other organisms, Escherichia coli effectively neutralizes the lethal effects of UVB when kept in the dark for 180 minutes.34
CONCLUSIONS
Medium-term exposure of Euglena gracilis cultures to solar UVB significantly reduces its motility. Therefore, this organism can be useful in field assays of biological dosimetry. In the field, E. gracilis is best suited for assays of 5-10 days of full sunshine. Although exposure to enhanced UVB resulted in reduced velocity in the laboratory, exposure to field conditions did not yield conclusive differences.
ACKNOWLEDGMENTS
This work was supported by EC grants EV5V-CT94-0425, and ENV4-CT96-0191 by DG-XII.
REFERENCES
- Molina, M.J., Molina, L.T. (1992) Stratospheric ozone. A. C. S. Symp. Ser. Am. Chem. Soc. Washington, D.C. 483, 24-35.
- Rontó, G., Gáspár, S., Gróf, P., Bérces, A. and Gugolya, Z. (1993) Ultraviolet dosimetry in outdoor measurements based on bacteriophage T7 as a biosensor. Photochem. Photobiol. 59: 209-214.
- Rontó, G., Gáspár, S. and Bérces, A. (1992) Phage T7 in biological UV dose measurement. J. Photochem. Photobiol. B Biol. 12: 285-294.
- Furusawa, Y., Suzuki, K. and Sasaki, M. (1990) Biological and physical dosimeters for monitoring solar UVB light. J. Radiat. Res. 31: 189-206.
- Regan, J. D., Carrier, W. L., Gucinski, H., Olla, B. L., Yoshida, H., Fujimura, R. K. and Wicklund, R. I. (1992) DNA as a solar dosimeter in the open ocean. Photochem. Photobiol. 56: 35-42.
- Tyrrell, R. M. (1978) Solar dosimetry with repair deficient bacterial spores. Action spectra, photoproduct measurements and a comparison with other biological systems. Photochem. Photobiol. 27: 571-579.
- Karentz, D. And Lutze, L. H. (1990) Evaluation of biologically harmful ultraviolet radiation in Antarctica with a biological dosimeter designed for aquatic environments. Limnol. Oceanogr. 35: 549-561.
- Munakata, N. (1974) Ultraviolet sensitivity of Bacillus subtilis spores upon germination and outgrowth. J. Bacteriol. 120: 59-65.
- Munakata, N. (1981) Killing and mutagenic action of sunlight upon Bacillus subtilis spores: a dosimetric system. Mutat. Res. 82: 263-268.
- Munakata, N. (1989) Genotoxic action of sunlight upon Bacillus subtilis spores: monitoring studies at Tokyo, Japan. J. Radiat. Res. 30: 338-351.
- Puskeppeleit, M., Quintern, L. E., El Inaggar, S., Schott, J. U., Eschweiler, U., Horneck, G. and Bücker, H. (1992) Long term dosimetry of solar UV radiation in Antarctica with spores of B. subtilis. Appl. Environm. Microbiol. 58: 2355-2359.
- Newton, J. W., Tyler. D. D. And Slodki, M. E. (1979) Effect of ultraviolet-B (280-320 nm) radiation on bluegreen algae (Cyanobacteria), possible biological indicators of stratospheric ozone depletion. Appl. Environ. Microbiol. 37: 1137-1141.
- Quintern, L. E., Horneck, G., Eschweiler, U. and Bücker, H. (1992) A biofilm used as ultraviolet-dosimeter. Photochem. Photobiol. 55: 389-395.
- Wang, T. V. (1990) A simple convenient biological dosimeter for monitoring solar UVB radiation. Biochem. Biophys. Res. Commun. 177: 48-53.
- Häder, D.-P. (1986) The effect of enhanced solar UVB radiation on motile microorganisms. NATO ASI Series, Vol. G8, 223-233.
- Blakesfield, M. K. and Calkins, J. (1991) Inhibition of phototaxis in Volvox aureus by natural and simulated solar ultraviolet light. Photochem. Photobiol. 55, 867-872.
- Victoria, D. and Häder, D.-P. (1991) Effects of solar and ultraviolet radiation on motility, photomovement and pigmentation in filamentous gliding cyanobacteria. FEMS Microbiol. Ecol. 86, 159-168.
- Petersen-Mahrt, S. K. , Ekelund, N. G. A. and Widell, S. (1994) Influence of UVB radiation and nitrogen starvation on daily rhythms in phototaxis and cell shape of Euglena gracilis