ELECTRON TRANSFER MECHANISMS IN PHOTOSENSITIZATION BY THE ANTI-INFLAMMATORY DRUG BENZYDAMINE
Douglas E Moore and Jian Wang
Department of Pharmacy, The University of Sydney, Sydney 2006
Australia
Abstract
The novel anti-inflammatory drug benzydamine has been shown to
photosensitize the reduction of Nitro Blue Tetrazolium,
ferricytochrome-c and copper (II) bathocuproinedisulphonate in
aqueous solutions (pH 7.4, 30oC) when irradiated with
UV light at its maximum absorption wavelength of 308 nm. The
reduction reactions all proceed most efficiently when the
solutions are deoxygenated, clearly indicating that direct
electron transfer occurs from the excited state of the sensitizer
to the substrate. In aerated solutions the reduction reactions
are slower and are partially inhibited by superoxide dismutase,
suggesting that superoxide anion could be involved as an
intermediate when oxygen is present. Benzydamine also
photosensitizes the oxidation of l-histidine and
2,5-dimethylfuran by the singlet oxygen pathway in aerated
solutions. The ability of benzydamine to participate as
sensitizer in several types of photochemical reaction is relevant
to the observed clinical photosensitivity of the drug.
1. Introduction
In addition to the direct responses to UVA and UVB exposure, the
human system can be subjected to sunlight-caused effects similar
to an exaggerated sunburn but mediated through exogenous
photosensitizers such as prescription medication. In general, the
generation of an adverse photosensitivity response can be
postulated to involve one or more of the pathways shown in Figure
1.
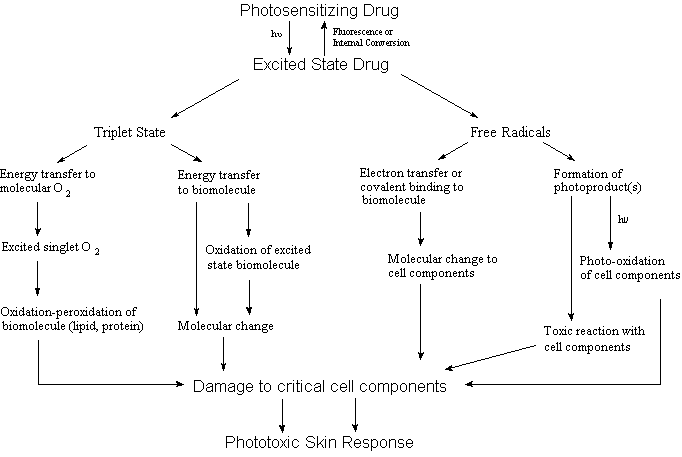 The
molecular mechanisms of photosensitization by drugs are being
investigated in our laboratory by studying the properties of
their photoexcited states, and free radical and singlet oxygen
mediated photooxidation reactions which ensue in model biological
systems. The photooxidation of susceptible biological substrates
by singlet oxygen or free radical pathways is widely believed to
lead to the initiation of the adverse responses. However, it is
possible that, in conditions of low oxygen concentration, or when
the sensitizer is located close to susceptible substrates, direct
electron transfer from sensitizer to substrate may become a
significant contributor. Oxygen may also be involved as an
electron carrier in the form of the superoxide anion radical.
The
molecular mechanisms of photosensitization by drugs are being
investigated in our laboratory by studying the properties of
their photoexcited states, and free radical and singlet oxygen
mediated photooxidation reactions which ensue in model biological
systems. The photooxidation of susceptible biological substrates
by singlet oxygen or free radical pathways is widely believed to
lead to the initiation of the adverse responses. However, it is
possible that, in conditions of low oxygen concentration, or when
the sensitizer is located close to susceptible substrates, direct
electron transfer from sensitizer to substrate may become a
significant contributor. Oxygen may also be involved as an
electron carrier in the form of the superoxide anion radical.
Nitro Blue Tetrazolium (NBT) and (ferri)cytochrome-c (Cyt) are reagents which are frequently used to detect the occurrence of the superoxide anion radical in an enzymic or photosensitized reaction [1, 2]. Superoxide can be formed by electron transfer from a donor to molecular oxygen but it is quenched by NBT or Cyt which are thereby reduced to diformazan and ferrocytochrome-c, respectively. The detection of superoxide is confirmed when addition of the enzyme superoxide dismutase (SOD) causes a decrease in production of diformazan from NBT. The possibility exists for direct electron transfer from the photosensitizer to NBT if its rate is competitive with that of superoxide formation. This is more likely when oxygen is in short supply.
Another compound which can be used for this test is the copper (II) complex with a bifunctional ligand such as bathocuproine disulphonic acid (BCDS) which shows SOD-like activity [3]. The electron transfer to the Cu(II)-BCDS complex resulting in reduction to the Cu(I) complex is detectable by a spectrophotometric change [4].
A number of photosensitizing drugs previously
tested in this laboratory [5-7] have been shown to undergo
photoionization and/or are active in free radical generation.
They may also participate in electron transfer processes. We have
already reported that 6-mercaptopurine reacts with NBT or
p-nitroso-dimethylaniline when irradiated in an oxygen-free
solution [5]. The antibacterial drug sulfamethoxazole was also
found to participate in photo-initiated electron transfer to NBT
and Cyt [7]. In this paper the use of NBT, Cyt and BCDS is
described for the investigation of electron transfer mechanisms
involving the drug benzydamine. This compound is a unique
non-steroidal anti-inflammatory agent which also possesses local
anaesthetic properties. However, many cases of photosensitivity
reactions following topical application or oral ingestion of
benzydamine have been reported [8-14]. Its chemical structure is
given with the UV absorption spectrum in Figure 2.
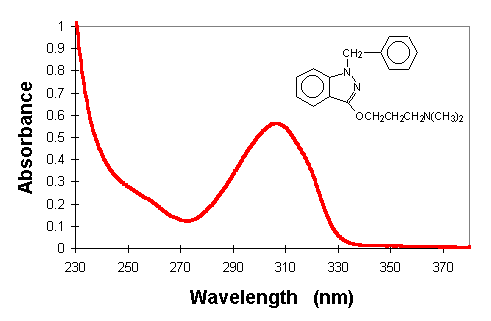
Figure 2. Structure and absorption spectra of benzydamine (1 x 10-4
M) in aqueous buffer solutions
2. Methods
Benzydamine hydrochloride was kindly supplied as the pure
substance by 3M Pharmaceuticals Pty Ltd, Sydney, and used as
received. Buffered aqueous solutions (phosphate, 0.05 M, pH 7.4)
containing Cyt (0.1 mg/ml) NBT (5.0 x 10-5 M) or BCDS
(0.1 mM) with 50 mM benzydamine were
flushed with gas (N2 or O2 as required) for
40 minutes before irradiating. The samples were contained in
stoppered cylindrical quartz vessels of 10 mm pathlength and
irradiated with a 400 W medium pressure mercury arc (Applied
Photophysics Ltd, UK) through a 2 mm Pyrex glass filter (Corning
O-53). The photoreduction of NBT was followed as a function of
the irradiation time by determining the increase in absorbance at
560 nm due to the diformazan product [1]. The extent of reduction
of Cyt was determined by measuring, as a function of irradiation
time, the differences in absorbance between the maximum at 550 nm
and the minimum near 535 nm [2]. The reduction of Cu(II)-BCDS was
monitored at 484 nm [4].
3. Results
3.1 Reduction of Nitro Blue Tetrazolium
When NBT in N2- or O2-flushed solution was
irradiated in the absence of a photosensitizer, no detectable
reaction occurred. With the addition of benzydamine in a N2-flushed
solution the reduction of NBT could be seen by the appearance of
the diformazan at 560 nm. Figure 3 shows the reaction
photosensitized by benzydamine as a function of time. The
reaction occurred to a lesser extent when an air-saturated
solution was employed under otherwise identical conditions, and
was completely inhibited when the solution was flushed with
oxygen before irradiation. Thus it appears that the reduction of
NBT photosensitized by benzydamine can be a direct reaction from
the excited state of the sensitizer to NBT which molecular oxygen
is able to inhibit.
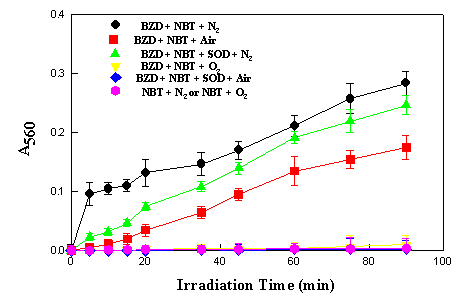 Figure 3.
Photoreduction of NBT (5.0 x 10-5 M) sensitized by
benzydamine (5.0 x 10-5 M) in aqueous buffer solution
pH 7.0 and 30oC
Figure 3.
Photoreduction of NBT (5.0 x 10-5 M) sensitized by
benzydamine (5.0 x 10-5 M) in aqueous buffer solution
pH 7.0 and 30oC
There are, however, a number of competing processes in which oxygen can be involved, such as singlet oxygen or superoxide formation. These were investigated as follows. Addition of SOD to the reaction mixture irradiated under air-saturated conditions completely inhibited the formation of diformazan, implying the involvement of superoxide in the reaction when some oxygen was present. The question arises as to why oxygen saturation inhibits the reaction completely when superoxide is diagnosed as being present under air-saturated conditions. It is suggested that the reaction is oxygen-concentration dependent. This behaviour is similar to that observed with 6-mercaptopurine [5] but different from that with sulfamethoxazole [7] or a-terthienyl [15]. However, all results are consistent with the report [16] that direct elevation of pO2 will suppress the photochemical reduction of NBT to formazan by simple mass action, as follows:
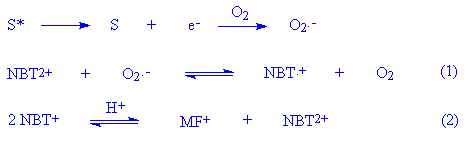
The first step in the reduction of NBT2+ to the
monoformazan MF+ by O2.- is the
production in (1) of the tetrazoinyl radical which then
disproportionates by reaction (2). The monoformazan then reacts
to the end product diformazan by the same sequence of reactions.
Addition of SOD shifts (1) to the left by decreasing the steady
state concentration of O2.- thereby
reducing the concentration of the tetrazoinyl radical and
subsequently the monoformazan. Another way of affecting reaction
(1) is by raising the pO2 level. Since oxygen is both
essential to the production of O2.- and is
an inhibitor of the reduction of NBT2+ there is a
range of pO2 which will be optimal for the
photochemical reduction of NBT2+ to the formazan. The
pO2 value in air-equilibrated solutions may be near
that optimum value, as suggested by irradiation of riboflavin,
NBT2+ and tetramethylethylenediamine [16].
The reduction of NBT induced by irradiated benzydamine under anaerobic conditions appears biphasic with a short rapid initial stage superimposed on a slower reaction. SOD diminished the extent of reduction only in this initial stage with little influence on the latter part. Similar results were also reported for photoreduction of NBT by riboflavin and methionine [17] and 6-mercaptopurine [5]. This result suggests that under anaerobic conditions, two mechanisms may be considered - either direct electron transfer from excited sensitizer to NBT, or initial expulsion of one electron from the excited sensitizer to the medium, and subsequent reaction of the solvated electron with the reagent. In both cases, the possibility that the reactions were actually due to one or more photoproducts could not be excluded, since the UV spectrum of benzydamine has changed after this period of irradiation time. All the above results implicate electron transfer from the excited states of benzydamine.
3.2 Cytochrome c reduction
Ferricytochrome c is an electron acceptor which differs from the
NBT reduction in that only one electron is required to reduce the
fully oxidized "ferri" form to the fully reduced
"ferro" form [18]. From the redox potentials for O2.-
[Eo (O2/O2.-) = -330
mv, E'o (O2.-, H+/H2O2)
= 940 mv] [19] and cytochrome c [Eo (FeIII
cytochrome c /FeII cytochrome c) = 260
mv] [20], it is evident that, on thermodynamic considerations
alone, O2.- could either oxidize or reduce
ferricytochrome c. In the presence of Na2S2O4
and O2, O2.- reduces
ferricytochrome c in 100 % yield [21]. After capturing one
electron, Cyt (III) will be reduced to Cyt (II) producing an
absorption band at 550 nm. The absorption spectra of oxidized and
reduced cytochrome c are very characteristic and distinct,
and this spectral change provides a sensitive and convenient
assay for an electron transfer reaction. It has been used to
detect the superoxide anion in aerated condition [22, 23] and
electron release from a sensitizer in deaerated conditions. Such
electron transfer has been demonstrated with benzo[a]pyrene [24], a-terthienyl
[15], anthracene [25],
2-chloro-3,11-tridecadiene-5,7,9-triyn-1-ol [26] and
sulfamethoxazole [7]. When oxygen- or air- or nitrogen- saturated
samples containing benzydamine and cytochrome c were kept
in the dark or when cytochrome c alone was irradiated, no
spectral changes were observed. When the experiments were
performed under continuous irradiation in a oxygen-saturated
solution containing benzydamine 5.0 x 10-5 M and
cytochrome c (0.1 mg/mL) in pH 7.4 phosphate buffer, there
was an increase in absorbance at 550 nm. The difference in
absorbance (A550 - A535 ) plotted as a
function of irradiation time in Figure 4, shows a steady increase
with time, reaching a plateau after about 10 min irradiation.
However by adding sodium hydrosulfite to the irradiated medium, a
further increase in the absorbance of the 550 nm peak was
observed, with conversion of all the Cyt (III) to Cyt (II). This
means that cytochrome c was not the limiting reagent.
There appear to be other factors inhibiting this reduction
process. One possibility is that cytochrome c itself
introduces an inner filter effect by light absorption at 365 nm,
although this usually happens at cytochrome c
concentrations higher than 0.1 mg/mL [15]. Upon addition of SOD
(300 units/mL) which competes with Cyt (III) for reaction with
superoxide anion, the reduction was totally suppressed, implying
that photoreduction of cytochrome c induced by benzydamine
is a photodynamic process, in which the superoxide anion radical
was involved in the photoinduced transfer of an electron to
cytochrome c. This result is similar to that described for
a-terthienyl [15], in which the growth
of the 550 nm band due to electron transfer induced by a-terthienyl to cytochrome c
increased until it reached a constant value.
When the SOD experiment was carried out with benzydamine under air-saturated conditions, the extent of cytochrome c reduction decreased, but showed the same trend as observed under oxygen-saturated conditions. This suggests there may be intermediates involved in addition to superoxide anion. In fact, benzydamine can also participate in the photoreduction of cytochrome c by a mechanism which does not require oxygen. Under anaerobic conditions, cytochrome c photoreduction induced by benzydamine was demonstrated to take place at a rate faster than for cytochrome c alone, but slower than the photosensitized reduction under aerobic conditions, suggesting a direct reaction with cytochrome c. These results are similar to those reported with benzo[a]pyrene [24] and anthracene [25] in which the photoreduction was greater in the presence of oxygen than in its absence, and the photoreduction in the presence of oxygen was greatly diminished by addition of the enzyme SOD, indicating superoxide anion involvement.
The results obtained aerobically in the presence and absence of SOD prove that electronically excited benzydamine preferentially transfers electrons to cytochrome c using oxygen as an intermediate, even though the reduction can take place in its absence. This is probably because the dissolved oxygen concentration in air saturated water is 235 mM at 30oC [27] representing a large molar excess over cytochrome c (8 mM) in the environment of each electronically excited molecule of benzydamine.
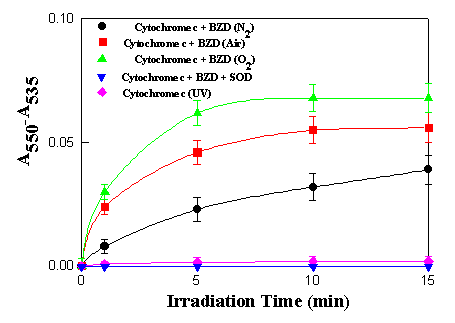 Figure 4. Photoreduction of cytochrome c (0.1 mg/mL)
sensitized by benzydamine (5.0 x 10-5 M). Each value
shown is the average of three determinations
Figure 4. Photoreduction of cytochrome c (0.1 mg/mL)
sensitized by benzydamine (5.0 x 10-5 M). Each value
shown is the average of three determinations
3.3 Reduction of Copper(II) complex with SOD-like activity
The knowledge that the enzyme Cu/Zn-SOD controls superoxide via
disproportionation of O2.- into O2
and H2O2 suggests that some other compounds
which are known as efficient catalysts of the dismutation process
can be used to replace SOD to detect the involvement of electron
transfer. For example, copper(II) is able to capture electrons in
the presence of a complexing agent such as
bathocuproinedisulphonic acid disodium salt hydrate (BCDS) which
stabilizes the reduced copper as a copper(I) complex. The
reaction course can be monitored by spectrophotometry at 484 nm
which is the maximum absorption of the complex Cu(I)BCDS [4]. The
SOD-like activity of the copper system with BCDS has been
verified by an indirect method using cytochrome c assay.
When Cu(II)Cl2, BCDS and benzydamine were mixed with
cytochrome c under oxygen-saturated conditions, the
reduction of cytochrome c was completely suppressed.
When benzydamine 1.0 x 10-4 M in phosphate buffered saline (PBS) solution was mixed with 0.1 mM BCDS and 30 mM copper sulphate, and irradiated both under aerobic and anaerobic conditions, an absorption change at 484 nm was observed. It was found that, under N2, the reaction goes slightly faster than under O2. The absorption increased with the time of irradiation until all the copper (II) was transformed to the copper(I) complex (Figure 5).
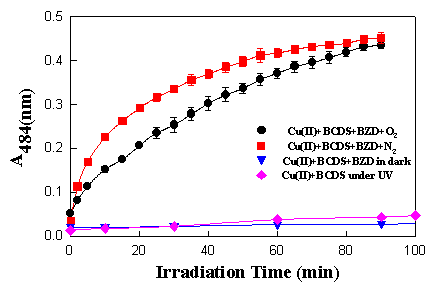
Figure 5. Formation of copper(I) BCDS complex induced by benzydamine (1 x 10-4 M) as a function of irradiation time at pH 7.4 and 30oC
When Cu(II) and BCDS were irradiated without benzydamine or the solution of benzydamine and Cu(II) and BCDS was kept in the dark, no absorption at 484 nm was observed after 100 min. This result provided further confirmation that an electron transfer reaction induced by benzydamine had occurred. The slower rate in the presence compared to the absence of oxygen could be due to oxygen quenching the excited state of benzydamine. This is consistent with the results obtained through experiments with NBT and cytochrome c.
3.4 Singlet oxygen mediated photooxidation
In separate experiments, benzydamine was found to photosensitize
the oxidation of l-histidine or 2,5-dimethylfuran in
air-saturated solutions. These compounds are substrates for
singlet oxygen, and although they are not completely specific, a
firm indication of the participation of singlet oxygen was found.
The photooxidation reaction was followed by measuring the depletion of oxygen with a Clarke-type oxygen electrode [28]. Figure 6 shows the effect of increasing concentration of benzydamine on the oxygen uptake rate of pH 7 buffered solution in the presence and absence of substrates when irradiated by the medium pressure mercury arc through a glass filter. Each result is the mean of triplicate measurements. In the presence of DF, or histidine, increased oxygen uptake was observed. The rate of oxygen consumption increased linearly at low benzydamine concentrations, then the rate gradually approached a plateau value, implying that all the radiation has been absorbed, and the light intensity becomes rate determining. No oxygen uptake was detected when DF or histidine was irradiated in the absence of drug. When benzydamine was added into the solution and kept out of the light, there was no detectable dark reaction. Upon irradiation, benzydamine was itself oxidized, the extent increasing with increasing concentration. At each concentration, the oxygen uptake rate was linear with time, i.e., zero order, indicating that the absorption of light was the rate-limiting factor. The participation of singlet oxygen was also indicated by the fact that addition of 0.01 M azide ion, a 1O2 quencher in aqueous solutions [29], reduced the photooxidation rate by 75%.
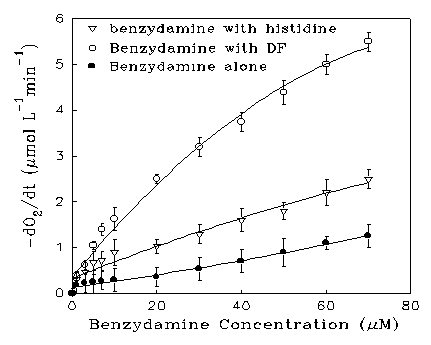 Figure 6. Oxygen uptake rates as a function of benzydamine
concentration at pH 7 and 30oC using 2,5-dimethylfuran
(DF, 2 mM) and histidine (2 mM) as substrates
Figure 6. Oxygen uptake rates as a function of benzydamine
concentration at pH 7 and 30oC using 2,5-dimethylfuran
(DF, 2 mM) and histidine (2 mM) as substrates
Variation of pH of the solution showed that the cation form of benzydamine (pKa 9.2) is 3-fold more efficient as a photosensitizer than the neutral form. Although the site of ionization in benzydamine is the alkylamino group which is distant from the absorbing chromophore, the difference in oxygen uptake capability between the base and its conjugate acid shows that the positive charge on the molecule is favourable for energy transfer to molecular oxygen. This is a similar pH dependence to that of the fluorescence yield of benzydamine and suggests that the free amino group quenches the excited state to some extent.
4. Conclusions
When benzydamine is irradiated in aerated solutions competing
pathways of photosensitization are possible, namely, singlet
oxygen mediated oxidation, and electron transfer mechanisms. The
relative importance of each would be dependent of the presence of
the relevant substrates near to the photoexcited benzydamine
molecule. The electron transfer process is more likely to be an
important factor in the photobiological activity of benzydamine
in conditions of low oxygen concentrations.
5. References
[1] Kirby T.W. and Fridovich I. (1982) A picomolar spectrophotometric assay for superoxide radical. Anal. Biochem., 127: 435-440.
[2] Auclair C. Torres M. and Hakim J. (1978) Superoxide anion involvement in NBT reduction catalysed by NADPH-cytochrome p-450 reductase. FEBS Lett., 89: 26-28.
[3] Zak B. (1958) Simple procedure for the single sample determination of serum copper and iron. Clinical Chemstry Acta, 3: 328-332.
[4] Costanzo L.L. De Guidi G. Guiffrida S. Rizzarelli E. and Vecchio G. (1993) Determination of SOD-like activity of copper(II) complexes. J. Inorg. Biochem., 50: 273-281.
[5] Hemmens V.J. and Moore D.E. (1986) Photochemical sensitization by azathioprine and its metabolites-I. 6-mercaptopurine. Photochem. Photobiol., 43: 247-255.
[6] Moore D.E. and Chappuis P.P. (1988) A comparative study of the photochemistry of the non-steroidal anti-inflammatory drugs. Naproxen, benoxaprofen and indomethacin. Photochem. Photobiol., 47: 173-181.
[7] Zhou W. and Moore D.E. (1997) Photosensitizing activity of the anti-bacterial drugs sulfamethoxazole and trimethoprim. J. Photochem. Photobiol., B. Biology, 39: 63-72.
[8] Ikemura I. (1971) Contact and photocontact dermatitis due to benzydamine hydrochloride. Japanese J. Clin. Dermatol., 25: P 129.
[9] Fernandez De Corres L. (1980) Photodermatitis from benzydamine. Contact Dermatitis, 1980, 6: 285-303.
[10] Balato N. Lembo G. Patruno C. and Ayela F. (1986) Contact dermatitis from benzydamine hydrochloride. Contact Dermatitis, 15: 105.
[11] Bruynzeel D.P. (1986) Contact allergy to benzydamine. Contact Dermatitis, 14: 313-314.
[12] Motley R.J. and Reynolds A.J. (1988) Photodermatitis from benzydamine cream. Contact Dermatitis, 19: p 66.
[13] Foti C. Vena G.A. and Angelini G. (1992) Occupational contact allergy to benzydamine hydrochloride. Contact Dermatitis, 27: 328-329.
[14] Goday B.J.J. Ilardia L.R. and Soloeta A.R. (1993) Allergic contact dermatitis from benzydamine with probable cross-reaction to indomethacin. Contact Dermatitis, 28: 111-112.
[15] Kagan J. Bazin M. and Santus R. (1989) Photosensitization with a-terthienyl: the formation of superoxide ion in aqueous media. J. Photochem. Photobiol., B: Biology, 3: 165-174.
[16] Clare D.A. Duong M.N. Darr D. Archibald F. and Fridovich I. (1984) Effects of molecular oxygen on detection of superoxide radical with nitroblue tetrazolium and on activity stains for catalase. Anal. Biochem., 140: 532-537.
[17] Beauchamp C. and Fridovich I. (1971) Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal. Biochem., 44: 276-287.
[18] Bensasson R.V. Land E.J. and Truscott T.G. (1983) Flash Photolysis and Pulse Radiolysis. Contributions to the Chemistry of Biology and Medicine, Pergamon Press, Oxford, p 155.
[19] Muir Wood P. (1974) The redox potential of the system oxygen - superoxide. FEBS Letters, 44: 22-24.
[20] Margalit R. and Schejter A. (1973) Cytochrome c: a thermodynamic study of the relationships among oxidation state, ion-binding and structural parameters. 1. The effects of temperature, pH and electrostatic media on the standard redox potential of cytochrome c. European J. Biochem., 32: 492-499.
[21] Butler J. Jayson G.G. and Swallow A.J. (1975) The reaction between the superoxide anion radical and cytochrome c. Biochim. Biophys. Acta, 408: 215-222.
[22] Kirby T.W. and Fridowich I. (1982) A picomolar spectrophotometric assay for superoxide radical. Anal. Biochem., 127: 435-440.
[23] Picker S.D. and Fridovich I. (1984) On the mechanism of production of superoxide radical by reaction mixtures containing NADH, Phenazine methosulfate, and nitroblue tetrazolium. Arch. Biochem. Biophys., 228: 155-158.
[24] Kagan J. Tuveson R.W. and Gong H.-H. (1989) The light-dependent cytotoxicity of benzo[a]pyrene: effect on human erythrocytes, Escherichia coli cells and Haemophilus influenzae transforming DNA. Mutation Res., 216: 231-242.
[25] Tuveson R.W. Wang G.R. Wang T.P. and Kagan J. (1990) Light-dependent reactions of anthracene. Photochem. Photobiol., 52: 993-1002.
[26] Kagan J. Wang T.P. Kagan I.A. Tuveson R.W. Wang G.R. and Lam J. (1992) Photosensitization by 2-chloro-3,11-tridecadiene-5,7,9-triyn-1-ol: damage to erythrocyte membrane, Escherichia coli, and DNA. Photochem. Photobiol., 55: 63-73.
[27] International Critical Tables (1928) Vol. III, P 262.
[28] Moore D.E. and Burt C.D. (1981) Photosensitization by drugs in surfactant solutions. Photochem. Photobiol., 34: 431-439.
[29] Spikes J.D. (1989) Photosensitization. In The Science of Photobiology, Second Edition. Edited by Smith K.C., Plenum Press, New York, 79-110.