EFFECTS OF Ca++ AND K+ ON MOTILITY AND PHOTOMOTILITY OF THE MARINE CILIATE FABREA SALINA
Spartaco Puntoni, Roberto Marangoni, Domenico Gioffrè and Giuliano Colombetti
Istituto di Biofisica del C.N.R., Via S. Lorenzo
26, 56127 Pisa, Italy
![]()
Abstract
Fabrea salina is a motile ciliate marine protozoan belonging to the order
Heterotrichida, that shows photomotile reactions. We report here the results of a study on
motility and phototaxis of F. salina exposed to different Ca++ and K+
concentrations. Our findings show that concentrations of potassium ions greater than that
of the growth medium and concentrations of calcium ions lower than that of the growth
medium cause a significant increase in phototactic response, while the cell speed is not
significantly affected. A more thorough analysis shows that the absolute concentrations of
Ca++ or K+ are not important and that the phototactic responsiveness depends mainly on the
ratio of the K+ concentration to the square root of the Ca++ (the so called Ja value), in
agreement with the results obtained in other protozoa. Our results are tentatively
discussed in terms of altered membrane potential or ion channel conductances.
![]()
1. Introduction
The motile behavior of ciliated protozoa is strongly influenced by membrane potential and
this, in its turn, depends on the amount of monovalent and divalent cations in the
suspension medium [1,2,3,4,5]. For instance, when Paramecium is transferred from
its adaptation medium to higher K+ concentrations it shows a period of ciliary reversal
[6,7,8,9], the duration of which depends on the Ja value, defined as the ratio of K
concentration to the square root of Ca concentration [7,8,9,10,11,12,13]. Iwatsuki and
Song [14] and Iwatsuki and Kobayashi [15] have shown that this is true also in Stentor
coeruleus. These authors have shown, furthermore, that the latency of the step-up
response in this ciliate is determined by the Ja value [15], as are the phototactic
orientation (which increases by increasing Ja values) and the percentage of cells showing
photophobic response (which increases by decreasing Ja values) [14]. Fabrea salina
Henneguy (1889) is a light-sensitive marine ciliate, which belongs to the order
Heterotrichida, like Blepharisma japonicum and Stentor coeruleus. Like these
ciliates, it shows photomotile responses, but, whilst Blepharisma japonicum only
shows step-up photophobic responses [16] and Stentor coeruleus negative phototaxis
and step-up photophobic responses, Fabrea salina exhibits positive phototaxis and
photophobic step-down reactions [17,18]. The experimental evidence available so far
suggests that membrane potential may play an important role in the mechanism of
photomotile reactions of Stentor coeruleus and Blepharisma japonicum
[15,19,20]. We have therefore investigated the phototactic response of Fabrea salina
as a function of the ionic concentrations to which the cells are exposed, with attention
given to the role of K and Ca ions. Our results indicate that the phototactic response
does not depend on the absolute concentrations of K and Ca, but that the parameter that
better correlates with the alteration of phototactic sensitivity is the Ja value, as
defined above.
![]()
2. Materials and methods
Fabrea salina were grown in artificial sea water (density 1.022) at a constant
temperature of 22 C in a light-dark cycle (15 h light-9 h dark). F. salina were fed the
green marine alga Dunaliella salina, which was grown in the growth medium suggested
by Johnson [21] and on the same light-dark cycle. D. salina used to feed F. salina were
centrifuged and washed three times in the ciliate growth medium. F. salina cells
were starved for four days before being used for the experiments. F. salina has a
pear-shaped body, the dimensions of which range from 100 to 300 mm for the long axis and
from 50 to 200 mm for the short axis. The cell surface is very densely covered with cilia:
somatic and oral cilia are clearly distinguishable. The adoral zone is characterized by a
deep spiral-shaped invagination, on the edge of which adoral membranelles are visible. F.
salina swims along a left-handed helix with an average speed of 200-300 mm/s (at 24
C) and shows two kinds of responses to light stimuli: positive phototaxis (cell
displacement towards the light source) and a photophobic step-down reaction (upon a sudden
decrease in light fluence rate the cells stop, turn and start swimming again in a new
direction) [17,18]. Fabrea salina cells were collected and concentrated from
cultures starved for four days; part of the cells, used as control, was resuspended in a
medium with the following composition: NaCl 300 mM, MgCl2 67 mM, CaCl2 14 mM, KCl 9 mM,
Na2SO4 31 mM, NaHCO3 2 mM and the pH adjusted to 8 with approximately 5 mM Tris- HCL; the
cells to be the tested were resuspended in media with different calcium and/or potassium
ions concentrations. The range of ionic concentrations investigated varied from 9 mM to 90
mM for potassium and from 1.75 to 56 mM for calcium. Ca-concentrations lower than 1.75 mM
and higher than 60 mM resulted in a complete arrest of motility, followed by cell death.
The same was true for K concentrations higher than 100 mM. Immediately after transfer to
the new medium, the cells usually showed a prolonged period of tumbling and backward
swimming, which lasted for some hours, depending on the different ionic composition of the
resuspension media. In order to allow the cells to completely adapt to the new ionic
conditions, we left them overnight in the new media; the next day we examined the cells
and considered them adapted and suitable for the experiments only when at least 70% of
them were motile and their speed was not significantly different from that of the control
samples (about 10% of the control value). In each experiment about 500 ml of cell
suspension containing 150-200 cells, the maximum number that we can simultaneously track,
were placed in the experimental chamber, the dimensions of which are 15mm*10mm*4mm. Cell
motion parameters (average speed, frequency of directional changes and frequency
distribution of the angles of directional changes) and phototactic reaction were measured
in control and treated samples. The measurements were performed by means of a laboratory
developed automatic tracking system, described in detail in a previous paper (Marangoni et
al., 1996). Phototaxis stimulating light was provided by means of a 150W Xenophot HLX
lamp; its wavelength was selected by means of a B579 filter (Balzers, Germany) with
maximum transmission at 579 nm and band width at half maximum of about 10 nm; the light
stimulus was kept on for three min at a fluence rate of 9 W/m2 . These conditions induce a
significant phototactic response in non- treated samples, as described in detail in a
previous paper [22]. The phototactic coefficient thus measured was corrected for
variations in cell speed [23] and normalized with respect to the phototactic
responsiveness of the control samples, taken arbitrarily equal to 1. Each experiment was
repeated four times and the data shown are the average of the values obtained together
with their standard deviation.
![]()
3. Results
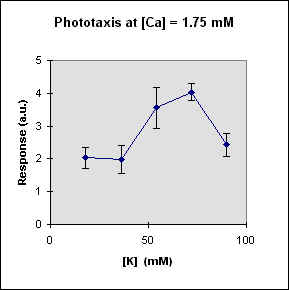
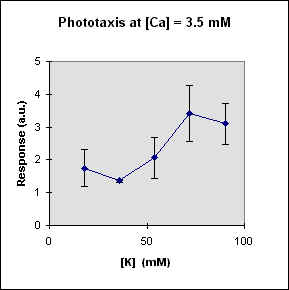
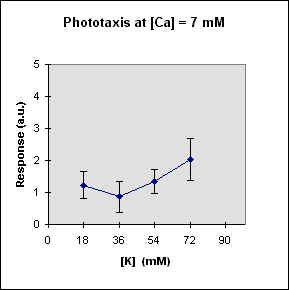
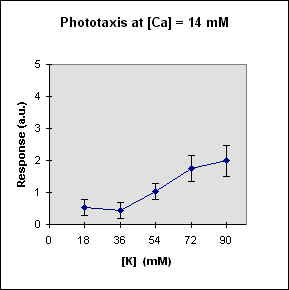
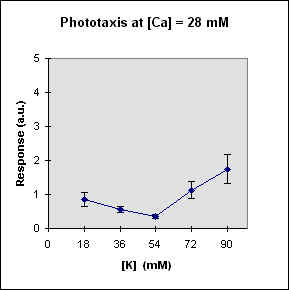
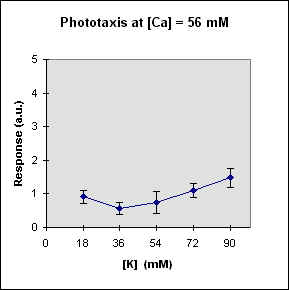
Fig 1 reports the results of a series of experiments in which we have varied the
K-concentration at different Ca concentrations.
![]()
 |
 |
 |
 |
 |
 |
![]()
A general feature of these results is that at each Ca concentration an increase in [K] brings about a meaningful increase in phototactic response. This increase is, however, also dependent on the Ca-concentration and is stronger at the lowest Ca concentration used. In this case, moreover, at high K potassium concentration the phototactic response shows a net decrease. Within the general trend of an increase in phototaxis with increasing [K], we can, moreover, observe a general slight decrease for [K] = 36 mM.
The effect of an increase in [K] on cell speed is less clear and, if any, it seems to depend also on the amount Ca present, as shown in Fig. 2.
![]()
Fig. 2 The average cell population speed at the various ionic compositions used. The different curves refer to the different Calcium concentrations used, indicated by the box inside. Abscissa: potassium concentration (mM). Ordinate: average sample speed relative to that of the control sample.
![]()
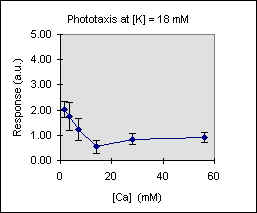
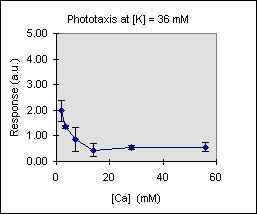
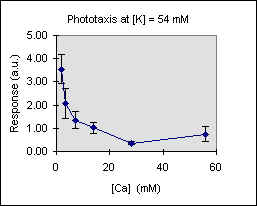
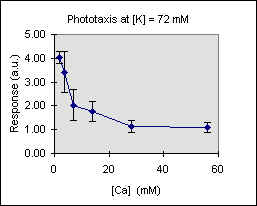
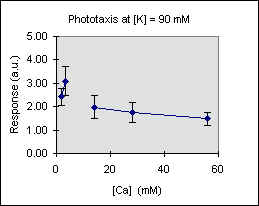
Figure 3 shows the trend of the phototactic response at fixed potassium concentration as a function of [Ca]. There is a general decrease of phototaxis, which is more marked when the phototactic responsiveness is higher, as, for example, at [K] = 54 mM..
![]()
 |
 |
 |
 |
 |
![]()
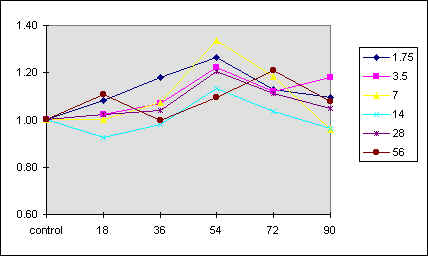
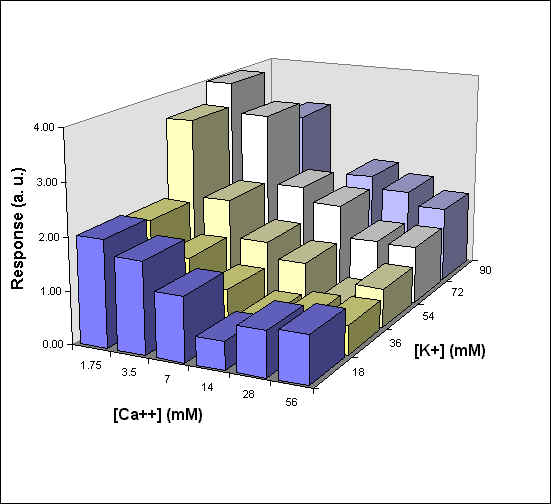
Figure 4 reports a 3D graph, which summarizes the behavior of phototaxis as a function of both [K] and [Ca] concentrations. In control experiments we have varied the ionic strength of the medium but kept [K] and [C] constant. In these conditions there was no measurable effect on the phototactic response of F. salina (data not shown).
![]()
Fig. 4 3D diagram of the phototactic response vs. [K] and [Ca] concentrations
![]()
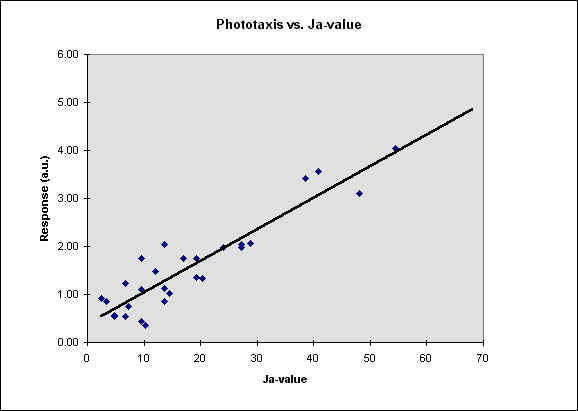
In figure 5 we report our results as a function of the Ja-value, defined as the ratio between [K] and the square root of [Ca].
![]()
Fig. 5 The phototactic response vs. the Ja-value. The r^2 is
about 0.84, then p is less than 0.05.
![]()
4. Discussion
All the available experimental evidence strongly indicate a close coupling between
membrane potential and motile responses mediated by the cilia. It is, therefore, not
surprising that it is possible to influence the photomotile reactions by changing the
ionic environment in which the cells are suspended. Iwatsuki and Song [14] have found
results similar to ours in the case of Stentor coeruleus. In this protozoan an
increase in [K] at a certain Ca concentration brings about a meaningful increase in the
phototactic response, whilst an increase in [Ca] at fixed [K] causes a significant
decrease in phototaxis. These authors suggest that the behavioral response to the light
stimulus might be linked not to the absolute [K] and/or [Ca], but rather be affected by
the so-called Ja-value. However, these authors weaken their hypothesis, by suggesting that
an increase in [Ca] can diminish the phototactic response of Stentor, for a
determined Ja value. In fact their conclusion goes beyond their experimental evidence,
which only shows that a simultaneous increase in [K] and [Ca] (necessary in order to keep
the Ja value constant) is accompanied by a decrease in phototaxis. The results shown in
Fig. 1 indicate that by keeping [Ca] constant and increasing [K] we can increase the
phototactic response of Fabrea salina, whereas an increase in [Ca] at constant
[K] brings about a strong decrease in phototactic sensitivity of the cells. We have then
asked the question whether Ca and K were able to affect the phototactic response t
independently of each other, or if there was a sort of competition between the two ions,
as suggested, for example, by Colombetti et. al [24] in Stentor. These authors
had suggested that the relative ratio [K]/Sqrt([Ca]) might be involved in the regulation
of the capability of Stentor to respond to light stimuli, but were unable to
establish a more precise relationship between the ionic concentrations and the photomotile
response. Our results, shown in Fig. 5, indicate that there is a meaningful correlation
between Ja value and phototaxis in F. salina. The r2 value calculated from our data
is meaningful at the confidence level; this indicates that there is a strong dependence of
phototaxis on the combined action of [Ca] and [K], which assumes the character of an
almost functional relation. What remains to be understood is the step (or are the steps)
of the photosensory transduction chain of Fabrea salina where high Ja values can
induce an amplification, that is reflected in a the amplification of the phototactic
response (which can be as high as a factor of 4). There is little doubt that this effect
cannot be at the level of light absorption itself. It is difficult to imagine that a
rhodopsin, which has been suggested as a possible photoreceptor in F. salina [25],
may change its optical absorption properties simply by changing the relative ratio of K to
the square root of Ca in the suspension medium. In the same manner, if we assume that
phototaxis in Fabrea salina relies on a shading mechanisms, it is hard to think of
how changing the ionic composition of the medium could render the shading mechanism more
efficient. One could possibly imagine that, if all the molecules involved in the
transduction chain are located on the ciliary membrane, as is the case for the rods, by
changing the relative ratio of K to the square root of Ca one could make one or more of
the amplification steps more efficient for a surface charge effect. Probably it is more
reasonable to consider the final steps of the sensory transduction chains, where usually
the opening or closing of ion channels are involved. These events might be affected by a
different ionic environment, with a competition between K and Ca for cell membrane binding
sites and a possible, subsequent activation (or deactivation) of membrane channels, for
instance. With regard to this it may be interesting to recall that in Stentor
high Ja values inhibit the step-up photophobic reaction, which has been shown to be linked
to a membrane depolarization followed by the opening of Ca channels in the cell membrane
and a subsequent Ca action potential, leading to ciliary reversal. In this case high Ja
values could bring about block the opening of the channels responsible for depolarization.
Similar behavioral results have been observed (Colombetti et al., unpublished) for the
step- down reaction of F. salina, which appears to be inhibited at high Ja values.
Let us now assume that a "good" phototaxis requires a prolonged swimming towards
the light source, which implies that the cells should avoid random turns during their
motion. A random turn in a ciliated protozoa can take place whenever there is a
depolarization of the membrane potential, in the limit when there is a Ca action
potential. If it is true that high Ja values can inhibit or make more difficult these
action potentials, as seems to be suggested by the results for the step-up in Stentor
or the step-down in Fabrea salina, we have a mechanism that can explain the
enhanced phototactic reaction in both systems. A direct proof of this hypothesis requires
a measurement of the random membrane fluctuations in these ciliates under different Ja
values.
![]()
References
- H. Machemer, Frequency and directional responses of cilia to membrane potential changes in Paramecium, J. comp. Physiol., 92 (1974) 293-316.
- H. Machemer and J. De Peyer, Swimming sensory cells: electrical membrane parameters, receptor properties and motor control in ciliated Protozoa, Verh. Dtsch. Zool. Ges. (1977) 86-110.
- H. Machemer, Electromotor coupling in cilia, in H.C. Lüttgau (ed.), Membrane control of cellular activity, Fortschr. Zool./Progr. Zool., 33 (1986) 205-250.
- H. Machemer, Motor control of cilia, in H.-D. Görtz (ed.), Paramecium, Springer Verlag, Berlin, Heidelberg, New York, Tokyo, 1988, pp. 216-235.
- H. Machemer, Cellular behavior modulated by ions: electrophysiological implications, J. Protozool., 36 (1989) 463-487.
- Y. Naitoh, R. Eckert, Electrical properties of Paramecium caudatum: modification by bound and free cations, Z. vergl. Physiol., 61 (1968) 427-452.
- J.M. Doughty, Control of ciliary activity in Paramecium-I: modification of K+ induced ciliary reversal by temprerature and ruthenium red, Comp. Biochem. Physiol., 61C (1978) 369-373.
- J.M. Doughty, Control of ciliary activity in Paramecium-II: modification of K+ induced ciliary reversal by cholinergic ligands and quaternary ammonium compounds, Comp. Biochem. Physiol., 61C (1978) 375-384.
- J.M. Doughty, Control of ciliary activity in Paramecium-III: evidence for specific membrane binding sites for ions and cholinergic ligands, Comp. Biochem. Physiol., 63C (1979) 183-197.
- T.L. Jahn, The mechanism of ciliary movement. II. Ion antagonism and ciliary reversal, J. Cell. Comp. Physiol., 60 (1962) 217-228.
- E. Hildebrand, S. Dryl, Significance of Ca++ and K+ ions for the excitation of the protozoan membrane, Bioelectrochem. Bioenerg., 3 (1976) 543-544.
- C. Hook, E. Hildebrand, Excitation of Paramecium. A model analysis. J. Math. Biol., 8 (1979) 197-214.
- C. Hook, E. Hildebrand, Excitability of Paramecium and significance of negative surface charges. A model analysis. J. Math. Biol., 9 (1980) 347-360.
- K. Iwatsuki, P.S. Song, The ratio of extracellular Ca++ to K+ ions affect the photoresponses in Stentor coeruleus, Comp. Biochem. Physiol., 92 (1989) 101-106.
- K. Iwatsuki, Y. Kobayashi, The latency of the photophobic response in Stentor coeruleus depends upon the ratio of extracellular Ca++ to K+ ions, Comp. Biochem. Physiol., 100 (1991), 711-714.
- F. Lenci, F. Ghetti, Photoreceptor pigments for photomovements of microorganisms, J. Photochem. Photobiol. B (Biol.), 3 (1989) 1-16.
- G. Colombetti, R. Marangoni, H. Machemer, Phototaxis in Fabrea salina, Med. Biol. Environ., 20 (1992) 93-100.
- G. Colombetti, R. Bräucker, H. Machemer, Photobehavior of Fabrea salina: responses to directional and diffused gradient-type illumination, J. Photochem. Photobiol. B (Biol.), 15 (1992) 253-257.
- S. Fabczak, H. Fabczak, N. Tao, P.S. Song, Photosensory transduction in ciliates. I. An analysis of light-induced electrical and motile responses in Stentor coeruleus, Photochem. Photobiol., 57 (1993) 696-701.
- S. Fabczak, H. Fabczak, P.S. Song, Photosensory transduction in ciliates. III. The temporal relation between membrane potential and photomotile responses in Blepharisma japonicum, Photochem. Photobiol., 57 (1993) 872-876.
- M.K. Johnson, E.J. Johnson, R.D. McElroy, H.L. Speer, B.S. Bruff, Effects of salts on the halophilic alga Dunaliella viridis, J. Bacteriol., 95 (1968) 1461-1468.
- R. Marangoni, S. Puntoni, L. Favati and G. Colombetti, Phototaxis in Fabrea salina. I. Action spectrum determination, J. Photochem. Photobiol. B: Biol., 23 (1994) 149-154.
- R. Marangoni, A. Batistini, S. Puntoni, G. Colombetti, Temperature effects on motion parameters and the phototactic reaction of the marine ciliate Fabrea salina, J. Photochem. Photobiol. B (Biol.), 30 (1995) 123-127.
- G. Colombetti, F. Lenci, P.S. Song, Effects of K+ and Ca++ ions on motility and photosensory responses of Stentor coeruleus, Photochem. Photobiol., 36 (1982) 609-611.
- A. Podestà, R. Marangoni, C. Villani and G. Colombetti, A rhodopsin-like molecule on the plasma membrane of Fabrea salina, J. Euk. Microbiol., 41 (1994) 565-569.