PHOTOSENSITIZATION BY RIBOFLAVIN: INHIBITING EFFECT OF OXIDATION PRODUCTS
E.L.Lozovskaya and I.I.Sapezhinskii
Institute of Biochemical Physics,
Russian Academy of Sciences,
4 Kosygin Street, Moscow, 117977
e-mail: efrem@glas.apc.org
Introduction
Riboflavin is an important vitamin and well-known photosensitizer [1]. However, under some conditions, its photosensitizing action is affected by the inhibiting action of photooxidation products. We observed this phenomenon in the course of oxidation of glycyl-tryptophan (Gly-Trp).
Peptide Gly-Trp is a convenient model for study of protein photooxidation. Photooxidation of Gly-Trp is accompanied with chemiluminescence (CL).
The study was carried out in a model system based on the measurement of photoinduced CL in aqueous solution. Chemiluminescence occurs due to decay of one of the oxidation products, dioxetane (D). Decay of the dioxetane results in product (P) in excited state:
D ® P, P* ® P + hn CL
This product is presumably, kynurenin-type compound.
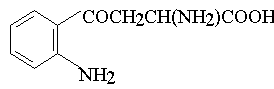
D, L - kynurenin
Methods.
Photochemiluminescence was measured with the specially designed set. The figure shows the scheme of measurement. The solution of Gly-Trp and riboflavin (phosphate buffer, pH 7.0) was poured into cuvette C1 and was irradiated with high pressure mercury lamp under stirring. Wavelength of 436 nm was cut off with glass filters. Immediately after irradiation a portion of solution was pumped into cuvette C2 of a high-sensitive photometric unit. Photocurrent of chemiluminescence was measured by recorder. The CL half-life was about 70 s at room temperature
CL decay kinetic curves were measured in continuous irradiation mode with pumping of small portion of solution into photometric unit every minute and pumping back 10 s after.
When Rose Bengal and erythrosine were used as sensitizers, solutions were exposed to light of 546 nm.
Kinetics of photosensitized CL
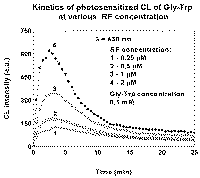
The decrease in CL intensity (Fig.1) during irradiation after the period of initial growth was found earlier in the case of direct irradiation of Gly-Trp solutions in UVA range without sensitizer [2]. Further investigation showed the similar effect for RF-sensitized CL.
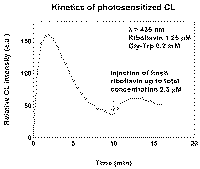
The effect is not due to sensitizer "fading" or Gly-Trp consumption: addition of fresh Gly-Trp or sensitizer portion slightly increases the intensity but does not restore initial CL level (Fig.2). The effect is more pronounced at higher RF concentrations (Fig. 1) as well as at higher Gly-Trp concentrations. Addition of small quantity of preliminary irradiated solution to experimental solution before irradiation caused marked decrease in the initial intensity.
The data obtained allow us to conclude that the decrease in CL intensity is due to inhibiting action of product(s) formed in the course of irradiation. This assumption is in a good agreement with concentration effects: the more are Gly-Trp and RF concentrations or light intensity, the higher is a product formation rate; hence, the stronger is inhibition.
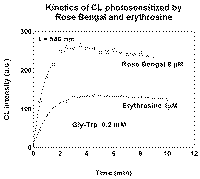
Inhibiting effect of products strongly depends on sensitizer nature. For example, no effect was observed when Rose Bengal (RB) or erythrosine were used as sensitizers (Fig. 3), although the inhibiting products were formed. It was shown by addition of a small portion of Gly-Trp solution, preliminary irradiated with RB, to fresh RF-containing Gly-Trp solution before irradiation. The CL intensity in this case was lower than in control experiment. It is clear that the difference between RF and RB is due to the difference in mechanisms of occurring of CL.
There are different primary processes resulting in CL. Under direct excitation of tryptophan chromophore photo-ionization takes place [1,3]. Peroxide radicals of Gly-Trp (RO2.) and superoxide are formed [4]. It is assumed that reaction between peroxide radical and superoxide results in dioxetane (D):
RO2. + O2-. ® D kr (1).
The latter decays with excited kynurenin-type product *P formation:
D ® P, *P kd (2).
This product is responsible for chemiluminescence emission.
In the case of sensitized CL the light is absorbed by sensitizer molecule. When triplet excited states are generated there are two possible pathways for CL. The first includes singlet oxygen reaction with Gly-Trp that results in dioxetane formation. Another pathway includes reaction of sensitizer in triplet excited state with Gly-Trp. Then in the presence of oxygen peroxide radical of Gly-Trp (RO2.) and superoxide are formed. The reaction of those results in dioxetane formation by reaction (1) as in the case of direct excitation. Of course, other ways, such as reactions of sensitizer in singlet excited state could not be excluded.
Riboflavin-sensitized CL
The CL pathway includes the following stages:
photo-initiation:
RF ® 3RF ® (+Gly-Trp, +O2) ® RO2., O2-. wi,
dioxetane formation by reaction (1) with reaction rate wD,
dioxetane decay by reaction (2).
Inhibiting effect of one (or a few) of oxidation products (In) is realized by reaction
In + O2-. ® ... kIn (3)
and maybe also by reaction with RO2.:
In + RO2-. ® ... ‘kIn (4)
This retards dioxetane formation as concentration of inhibiting product increases. Based on equation:
d[D]/dt = wD - kd[D]
when the influence of products is not accounted dioxetane concentration as well as CL intensity increases with time as
I ~ [D] ~ 1 - exp(-kdt).
If the rate of product formation is proportional to dioxetane concentration
d[P]/dt ~ [D]
then concentration of product depends on time as
[P] ~ kdt + exp(-kdt) - 1.
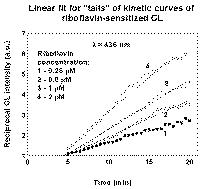
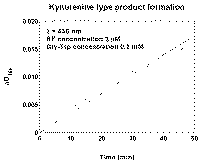
This dependance supposes initial delay and asimptote [P] ~ t at long times. The "tails" of kinetic curves from Fig. 1 fit to lines when plotted in coordinates Imax/I versus time (Fig. 4). It suggests that concentration of product-inhibitor linearly rises with time.
The experimental curves in Fig.1 could be sufficiently described as
![]()
where I0 is a limiting value of intensity without product influence, a is a parameter.
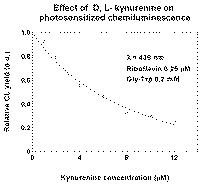
It is known that the main products of tryptophan oxidation are kynurenin derivatives. We studied the effect of DL-kynurenin on CL of Gly-Trp (with RF as sensitizer) [5]. Concentration of half-inhibition was equal to 5.10-6 M, rate constant for reaction of DL-kynurenin with superoxide was assessed as 1.10-7 M-1s-1 (Fig.5).
The oxidation rate in the experiments was too low to detect kynurenin by spectrophotometry. However, at higher irradiation doses we measured increase in absorbanse at 364 nm, corresponding kynurenin formation (Fig.6).
Conclusions
In the course of oxidation of peptide glycyltryptophan, photosensitized by riboflavin, some oxidation products, presumably kynurenin derivatives, act as inhibitors. The inhibiting effect is due to reaction of inhibitor with superoxide and/or peroxide radicals of peptide. Singlet oxygen oxidation is not affected, although inhibiting products are formed. The effect depends on riboflavin and peptide concentrations and exposure dose.
References
1. R.V. Bensasson, E.J. Land and T.G. Truscott. Flash Photolysis and Pulse Radiolysis. Pergamon Press, Oxford, 1983.
2. E.L. Lozovskaya, E.N. Makareyeva, I.V. Bolshakova and I.I. Sapezhinskii. Radiat. Biol. Ecol., 1996, Vol.36, Iss.3, pp.405-410 (In Russian).
3. D. Creed. Photochem. Photobiol., 1984, Vol.39, No. 4, pp.537-562.
4. I.I. Sapezhinskii and E.L. Lozovskaya. Chem. Phys. Repts., 1995, Vol. 14 (10), pp.1631-1656.
5. Ye.L. Lozovskaya and I.I. Sapezhinskii. Biophysics, 1993, Vol. 38, No.1, pp.25-29.