Light-induced, reversible modifications of the putative photoreceptor protein of Dictyostelium discoideum amoebae
THOMAS SCHLENKRICH, DONAT-P. HÄDER and MICHAEL LEBERT
Institut für Botanik und Pharmazeutische Biologie, Friedrich-Alexander-Universität, Staudtstr. 5, D-91058 Erlangen (Germany)
Correspondence to: Prof. Dr. Donat-P. Häder, Institut für Botanik und Pharmazeutische Biologie, Friedrich-Alexander Universität, Staudtstraße 5, D-91058 Erlangen, Fed. Rep. Germany.
Tel.: 49 9131 858216, Telefax: 49 9131 858215
Summary
Dictyostelium discoideum amoebae show positive phototaxis at low irradiances (< 0.1 W m-2) and negative phototaxis at higher intensities. Based on the action spectrum for photoaccumulation of amoebae we were able to identify a membrane-bound 45 kDa protein which we assume to be the photoreceptor and/or the antenna pigment for amoebal phototaxis. For further characterization polyclonal antibodies directed against the 45 kDa protein were generated. The amount of protein did not change with the growth conditions (light or dark grown amoebae), but the binding characteristics of the antibody to it’s antigen strongly depended on the availability of light. This finding indicates a light-induced, reversible modification of the putative photoreceptor. The nature of this modification is unknown.
Key words: Dictyostelium discoideum amoebae; phototaxis; photoreceptor pigment
INTRODUCTION
In the last few decades the eukaryotic cellular slime mold, Dictyostelium discoideum, has developed into a model system in which many different basic cellular processes can be studied at a molecular level. This is especially true for the field of physiology. The organism which was first described by Raper [1] has an interesting life cycle [2], in which single-celled amoebae undergo developmental changes leading to the formation of multicellular pseudoplasmodia, also called slugs because of their morphology.
Both amoebae and slugs use a number of external stimuli such as light, chemical and thermal gradients to orient themselves in their habitat [3, 4, 5]. The amoebae track down bacteria which are sensed using chemotactic orientation toward folic acid [6]. When the bacterial food is exhausted the amoebae start to aggregate following a cAMP gradient which leads to the formation of slugs [7]. The slugs consist of up to 100,000 individual cells which do not fuse, in contrast to acellular slime molds.
Amoebae and slugs can detect light and react to it. The action spectra for both stages in the life cycle differ significantly from each other [8, 9]. The action spectrum for slug phototaxis shows two peaks at about 420 and 440 nm, a broader maximum at 560 nm and a minor peak at 610 nm. The mechanism for the light perception is supposed to be based on a lens effect [5, 10]. The photoreceptor for slug phototaxis is still obscure. One hypothesis assumes the involvement of a flavin and a cytochrome b2 as chromophoric groups located on the same protein [11].
The assumption that slugs and amoebae use different photoreceptor systems for their phototaxis is not only supported by the two different action spectra but also by the fact that amoebae cannot use the lens effect because of their small size and highly variable form. Furthermore, a mutant was isolated lacking slug phototaxis, but showing amoebal phototaxis [12].
Amoebae show positive phototaxis at low irradiances (< 0.1 W m-2) [9], which changes to a negative one at higher irradiances [13]. Although the participation of multiple photoreceptors in amoebal phototaxis was discussed [14], we were able to isolate a single membrane-bound protein with an absorption spectrum that closely resembles the action spectrum for photoaccumulation of Dictyostelium discoideum amoebae [15, 16]. The aim of this paper is to further characterize this protein by using polyclonal antibodies.
MATERIALS AND METHODS
Organisms and culture conditions
The axenic strain AX2 of Dictyostelium discoideum was used throughout this study. The cells were grown in 100-ml Erlenmeyer flasks filled with 40 ml HL5-medium [17]. Streptomycin (250 µg ml-1) was added to prevent bacterial growth. Fresh flasks were inoculated with 1 ml of cell suspension in their late exponential phase (3 - 5 x 106 cells ml-1) and kept on a rotary shaker (125 rpm) for six days at 21°C in the dark.
Spectroscopy
The purified 45 kDa-protein for the spectroscopic measurements was obtained by using a two phase extraction method [18]. Absorption spectra were measured in a single beam spectrophotometer (DU70, Beckman, Palo Alto, USA) equipped with an Ulbricht“s sphere to compensate for the strong scattering of the sample. The spectra were measured in quartz cuvettes (Hellma, Müllheim, FRG) and buffer was used as a blank. The raw spectra were transferred to an IBM AT-compatible computer were they could be treated statistically and mathematically using the leader software (Beckman).
Fluorescence emission spectra were recorded with a spectrafluorimeter (RF-5000, Shimadzu, Kyoto, Japan) at room temperature. The evaluation was also performed on an IBM AT-compatible computer using the RF-PC software developed by Shimadzu.
Gel electrophoresis
SDS polyacrylamide gradient gel electrophoresis was carried out in a vertical system (2001, Pharmacia LKB) with gels of 140 x 110 mm, 1.5 mm thick using the method described by [19] with a gradient (8 to 20 % T) in the resolving gel. The samples contained the protein corresponding to 105 cells (100 µl medium) and were diluted with 50 µl of sample buffer (equal to a protein concentration of 1.2 mg ml-1 in each lane). The gels were Coomassie stained. To determine the molecular weight protein test mixtures 4 and 5 (Serva, Heidelberg, Germany) were co-separated.
Immunization of the rabbit
The 45 kDa protein was electroeluted from the SDS polyacrylamide gels using the Elucon-chamber of Biorad (Göttingen, Germany). Purity of this protein preparation was assessed by SDS-PAGE. The eluted protein was dialyzed against PBS (phosphate buffered saline, 10 mM potassium phosphate, pH 7.5, 0.9 % (w/v) NaCl). The dialyzed protein (100 µg in 1 ml PBS) was thoroughly mixed with 1 ml complete Freund“s adjuvant (Sigma, Deisenhofen, Germany) and injected subcutaneously into the back of a male albino rabbit. A booster injection of 100 µg protein in 1 ml incomplete Freund“s adjuvant was given 4 weeks later, a second booster injection with 50 µg protein was applicated intravenously two weeks after the first one. The animal was bled two weeks after the final booster injection and the serum was obtained. The serum was separated from whole blood by clotting and low speed centrifugation and stored in small aliquots at -20°C until used. Preimmune serum was collected by the same procedure prior to the initiation of the immunization schedule. The titer of the immune serum obtained was determined by a dilution series.
Western blotting and immunostaining
Aliquots of 100 µl medium (corresponding to 105 cells) were diluted with 50 µl sample buffer and incubated for 4 min at 90°C. After separating of the proteins on SDS PAGE as described above the gels were blotted for 3 h at 200 mA and 4°C on PVDF membranes (Millipore, Bedford, USA) using a semidry blotting technique (anode buffer: 50 mM CAPS, pH 10.0; cathode buffer: 50 mM CAPS, 0.1 % (w/v) SDS, pH 5.5).
Immunochemical staining of the transferred proteins was performed as follows; after saturation of the membranes with 5 % (w/v) BSA in TBS (Tris buffered saline, pH 7.5) overnight, the primary antibody was allowed to react in TBS/BSA for 30 min at room temperature. After washing with TBS and TBS/Triton X-100 (0.05 % (w/v)) the secondary antibody (anti-rabbit IgG, alkaline phosphatase conjugate, Sigma) was allowed to bind for 30 min. The blot was washed as above and the color was developed using BCIP/NBT tablets (Sigma).
40 ml AX2 amoebae cell suspension were kept in the dark until a density of 106 cells/ml was reached. The culture was irradiated with white light produced from a 250-W slide projector with a 24-V quartz halogen bulb (Kindermann Universal, Wetzlar, Germany) at 10-3 W m-2. After 0, 0.25, 0.5, 1, 2, 3 and 4 h of light exposure aliquots of 100 µl cell suspension were removed and separated by SDS PAGE.
Parallel to the dark-grown culture a second, light-grown culture was treated in the same way: after the light-grown amoebae had reached a cell density of 106 cells ml-1 they were kept in the dark. Aliquots were taken after 0, 0.25, 0.5, 1, 2, 3 and 4 h of darkness and subjected to SDS PAGE.
RESULTS AND DISCUSSION
As outlined above the absorption spectrum of the 45.5 kDa protein closely resembles the action spectrum for the photoaccumulation of Dictyostelium discoideum amoebae [9, 16]. However, the fourth peak at 650 nm which can be seen in the action spectrum is clearly missing in the absorption spectrum. When excited at a wavelength of 412 nm, the 45.5 kDa protein shows a strong fluorescence emission at 650 nm (Fig. 1). Although not proofed, one possible explanation could be that the 45.5 kDa protein acts as an antenna pigment, collecting the light energy and transmitting it to a yet not identified acceptor pigment which absorbs at 650 nm.
Based on the assumption that the action spectrum and the absorption spectrum of a given photoresponse must match, it was concluded that the 45.5 kDa protein must play an important role in light perception of Dictyostelium amoebae [15]. Furthermore, also pigment synthesis depends on the light conditions: dark-grown amoebae show a much more pronounced reddish color compared to light-grown cells. The nature of the chromophoric group of the 45 kDa-protein is still unclear. An involvement of cytochromes was excluded, because the protein did not show a specific heme staining [20, 21]. Analysis of the positions
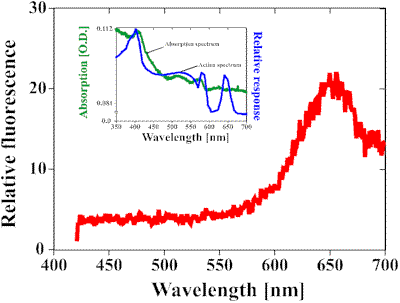 |
Fig. 1. The figure shows a fluorescence spectrum of the isolated 45 kDa protein of Dictyostelium discoideum amoebae (red line). The protein was excited at a wavelength of 412 nm, resulting in a fluorescence emission at a wavelength of 650 nm. The inset shows a comparison between the action spectrum of the amoebae of Dictyostelium discoideum (blue line) and the absorption spectrum of the 45 kDa protein (green line [16]). Both graphs show the same pattern with one exception: the peak at 650 nm which can be seen in the action spectrum, is clearly missing in the absorption spectrum. This led to the theory that the 45 kDa protein acts as an antenna pigment which collects the energy and then transfers it to a yet unknown acceptor which absorbs at 650 nm.
of the absorption maxima indicate that the chromophoric group is a protoporphyrin IX [22, 23]. This hypothesis is strongly supported by the close resemblance between the isolated protein-pigment complex and the chemical pure compound [15]. The fluorescence spectra also support this result [24, 23, 25]. To establish a connection between the higher pigment synthesis rate and the photoreceptor on the protein level, we used an immunological approach to study the function and structure of membrane proteins.
 Fig. 2:
Dilution series to determine relative sensitivity of the antibody raised against the 45.5
kDa protein. Each lane was loaded with Dictyostelium discoideum protein form 105 amaboe cells. For details see Material and Methods.
Fig. 2:
Dilution series to determine relative sensitivity of the antibody raised against the 45.5
kDa protein. Each lane was loaded with Dictyostelium discoideum protein form 105 amaboe cells. For details see Material and Methods.
Immunization of the rabbit with the highly purified, reduced and denatured 45.5 kDa protein resulted in the production of antibodies that reacted specifically with this protein. The titer obtained was determined using a dilution series (Fig. 2). The reaction of the immune serum with proteins from D. discoideum amoebae could be titrated over a range of serum dilution between 1:1000 and 1:8000. The preimmune serum tested under identical conditions did not react with Dictyostelium proteins.
Figure 3 shows that irradiation of Dictyostelium amoebae with white light leads to a decreased binding of the antibody to its
 Fig. 3. Immune
labelling of electrotransferred D. discoideum amoebae proteins by the anti-45.5
kDa-serum. Whole cells of D. discoideum amoebae were incubated with sample buffer
and subjected to SDS PAGE. After transferring the proteins onto PVDF membrane, immune
reactivity was visualized by incubating the washed membranes with anti-IgG-alkaline
phosphatase conjugate (1:5000 dilution), followed by reaction with the chromogenic
phosphatase substrate described in Materials and Methods.
A section of the membrane was stained with Coomassie brilliant blue. The cells were dark
grown and then irradiated with white light as discussed in Material
and Methods. Aliquots were removed after time periods indicated in the text. Each lane
contains a protein concentration which corresponds to approximately 105 cells.
Fig. 3. Immune
labelling of electrotransferred D. discoideum amoebae proteins by the anti-45.5
kDa-serum. Whole cells of D. discoideum amoebae were incubated with sample buffer
and subjected to SDS PAGE. After transferring the proteins onto PVDF membrane, immune
reactivity was visualized by incubating the washed membranes with anti-IgG-alkaline
phosphatase conjugate (1:5000 dilution), followed by reaction with the chromogenic
phosphatase substrate described in Materials and Methods.
A section of the membrane was stained with Coomassie brilliant blue. The cells were dark
grown and then irradiated with white light as discussed in Material
and Methods. Aliquots were removed after time periods indicated in the text. Each lane
contains a protein concentration which corresponds to approximately 105 cells.
antigen; after 4 h of irradiation only a faint band was visible on the immunoblots. In contrast, prolonged dark exposure led to an increase of the antibody binding to the 45.5 kDa protein (Fig. 4). The patterns for the increasing and decreasing of the antibody binding are similar; within minutes differences in the antibody-antigen binding can be detected. Thus, light-induced modifications of the 45.5 kDa protein take place, and these modifications seem to be reversible.
 Fig. 4. Immune
labelling of electrotransferred D. discoideum amoebae proteins from a light-grown
culture. Details as in Fig. 3. The cells were grown in light and then incubated in
darkness as discussed in Material and Methods. Aliquots were
removed after time periods indicated in the text. Each lane contains a protein
concentration which corresponds to approximately 105
cells.
Fig. 4. Immune
labelling of electrotransferred D. discoideum amoebae proteins from a light-grown
culture. Details as in Fig. 3. The cells were grown in light and then incubated in
darkness as discussed in Material and Methods. Aliquots were
removed after time periods indicated in the text. Each lane contains a protein
concentration which corresponds to approximately 105
cells.
The nature of the light-induced modification(s) is yet unknown. We did not detect a shift in the gel position of the 45.5 kDa protein, nor did we find any peptide fragments labelled with the antibody. Thus, an activation or inactivation of the putative photoreceptor synthesis and degradation of the protein must be excluded. The different binding capacity of the antibody could be due to light-induced folding of the protein. According to this hypothesis, the dark-adapted protein would offer more or easier accessible epitops to the antibody than the light-adapted protein.
It is also feasible that a yet unidentified regulatory mechanism controls the two different states of the photoreceptor. Similar mechanisms were described by Plangger et al. [26] for photoreceptors of fly eyes: they characterized a photoreceptor-specific protein that undergoes reversible binding to a light-activated photoreceptor, in this way controlling phosphorylation and dephosphorylation of the light-activated visual pigment.
In conclusion, we believe that the antibodies we have raised against the putative photoreceptor of Dictyostelium discoideum amoebae are useful in the further characterization of this specific protein. We are currently using these antibodies in studies designed to obtain more information on the localization of the 45.5 kDa protein within the cell.
ACKNOWLEDGEMENTS
This work was supported by financial aid from the Bundesminister für Forschung und Technologie (project KBF 57).
| [1] K.B. Raper, Dictyostelium discoideum, a new species of slime
mold from decaying forest leaves, J. Agr. Res., 50 (1935) 135-147. [2] J.T. Bonner, A descriptive study of the development of the slime mold Dictyostelium discoideum. Amer. J. Bot., 31 (1944) 175-182. [3] K.B. Raper, Pseudoplasmodium formation and organization in Dictyostelium discoideum. J. Elisha Mitchell Sci. Soc., 56 (1944) 241-282. [4] J.T. Bonner, W.W. Clarke, C.L. Neely, M.K. Slifkin, The orientation to light and the extremely sensitive orientation to temperature gradients in the slime mold Dictyostelium discoideum. J. Cell and Comp. Physiol., 36 (1950) 149-158. [5] D.W. Francis, Some studies on phototaxis of Dictyostelium. J. Cell and Comp. Physiol., 64 (1964) 131-138. [6] P. Pan, E. Hall, J.T. Bonner, Folic acid as a second chemotactic substance in the cellular slime molds. Nature, 237 (1972) 181-182. [7] G. Gerrisch, Chemotaxis in Dictyostelium. Ann. Rev. Physiol., 44 (1982) 535-552. [8] K.L. Poff, D.-P. Häder, An action spectrum for phototaxis by pseudoplasmodia of Dictyostelium discoideum. Photochem. Photobiol., 39 (1984) 433-436. [9] D.P. Häder, K.L. Poff, Light-induced accumulation of Dictyostelium discoideum amoebae. Photochem. Photobiol., 29 (1979) 1157-1162. [10] D.P. Häder, U. Burkart, Optical properties of Dictyostelium discoideum pseudoplasmodia responsible for phototactic orientation. Exp. Mycol., 7 (1983) 1-8. [11] K.L. Poff, W.L. Butler, Spectral characteristics of the photoreceptor pigment of phototaxis in Dictyostelium discoideum. Photochem. Photobiol., 20 (1974) 241-244. [12] D.P. Häder, B.D. Whitacker, K.L. Poff, Responses to light by a nonphototactic mutant of Dictyostelium discoideum. Exp. Mycol., 4 (1980) 382-385. [13] D.P. Häder, K.L. Poff, Photodispersal from light traps by amoebae of Dictyostelium discoideum. Exp. Mycol., 3 (1979) 121-131. [14] D.P. Häder, M. Lebert, Analysis of photoreceptor proteins of microorganisms by gradient gel electrophoresis and other biochemical separation methods. Electrophoresis, 15 (1994) 1051-1061. [15] H.-P. Vornlocher, D.-P. Häder, Isolation and characterization of the putative photoreceptor for phototaxis in amobae of the cellular slime mold, Dictyostelium discoideum. Bot. Acta, 105 (1991) 47-54. [16] T. Schlenkrich, P. Fleischmann, D.-P. Häder, Biochemical and spectroscopic characterization of the putative photoreceptor for phototaxis in amobae of the cellular slime mold, Dictyostelium discoideum. J. Photochem. Photobiol. B: Biol., 30 (1995) 139-143. [17] J.M. Ashworth, D.J. Watts, Metabolism of the cellular slime mold Dictyostelium discoideum in axenic culture. Biochem. J., 119 (1970) 175-182. [18] T. Schlenkrich, M. Porst, D.-P. Häder, A rapid, simple method for the isolation and characterization of the photoreceptor of Dictyostelium discoideum. FEBS Letters, 364 (1995) 276-278. [19] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227 (1970) 680-685. [20] M.R. Pudeck, P.D. Bragg, G. Weeks, Membrane bound cytochromes of the cellular slime mould Dictyostelium discoideum. FEMS Microbiol. Lett., 3 (1978) 123-125. [21] C. Woffendin, S.W. Edwards, A.J. Griffiths, The cytochromes of Dictyostelium discoideum. Comp. Biochem. Physiol., 75B (1983) 53-59. [22] K.M. Smith, General features of the structure and chemistry of porphyrin compounds. In: K.M. Smith, ed. Porphyrins and Metalloporphyrins, (1975) pp. 3-28, Elsevier, Amsterdam. [23] R.M.C. Dawson, D.C. Elliot, W.H. Elliot, K.M. Jones, Data for Biochemical Research (1986). Clarendon Press, Oxford. [24] S. Schwartz, M.H. Berg, I. Bossenmaier, H. Dinsmore, Determination of porphyrins in biological material. Meth. Biochem. Anal., 8 (1960) 222-293. [25] S. Nonell, M.L. Sesé, D.O. Mįrtire, S.E. Braslavsky, F.R. Trull, Polymer bound pyrrole compounds-VI. Photophysical properties of monomeric models for Polystyrene-bound porphyrins. Photochem. Photobiol., 53 (1991) 185-193. |