PERPLEXING ASPECTS OF THE TWO PHOTON CHEMISTRY OF ROSE BENGAL
Christopher R. Lambert*, Irene E. Kochevar** and Robert W. Redmond**
*Chemistry Department, Connecticut College, 270 Mohegan Avenue, New London, CT 06320
**Wellman Laboratories of Photomedicine, Massachusetts General Hospital and Harvard Medical School, Boston, MA 02114, U.S.A.
Correspondence: Christopher R. Lambert ..... crlam@conncoll.edu
Irene E. Kochevar ..... kochevar@helix.mgh.harvard.edu
Robert W. Redmond ... redmond@helix.mgh.harvard.edu
Keywords: photosensitization; rose bengal; non-linear photochemistry; two-photon
![]()
Abstract
Two photon laser flash photolysis has been used to investigate the upper excited state chemistry of rose bengal. The rose bengal triplet state, generated using 532 nm irradiation, has been monitored as a function of wavelength of the second photon. The second pulse is derived from a tunable OPO laser which allows for excellent selectivity in the excitation of the transient intermediate. It is found that the triplet state bleaches when only the triplet state absorbs the second photon however, no bleaching is observed when there is significant ground state absorption at the wavelength of the second photon. It is possible that the difference in the two experiments described, arises because of an experimental artifact or because the two photon chemistry is wavelength dependent.
![]()
Introduction
Photosensitized oxidation reactions may be categorized into those that are initiated by the action of singlet oxygen and those that are initiated by an electron or atom transfer process. These mechanisms have been classified as Type II and Type I photosensitizations respectively.
Singlet oxygen may be formed by energy-transfer from the lowest excited triplet state of a photosensitizer to ground state oxygen. Radical species may be generated, by excitation of a photosensitizer to higher energy upper excited states from which bond breakage or ionization may occur. Population of the upper excited state may be achieved by absorption of a UV-photon or, alternatively, simultaneous absorption of two visible photons. It is also possible to access a higher excited state by sequential absorption of two photons. Under these conditions, depending on the size of the absorption cross sections, if the lifetime of first excited singlet state is comparable to the delay in the timing of the two pulses, then the higher excited state may be populated.
The sequential absorption of two photons to populate higher excited states of organic molecules is well documented. Using a single laser wavelength enhanced sensitizing efficiency under high intensity excitation will only be observed when S1 or T1 absorbs at the wavelength used to excite the ground state of the dye and when Sn or Tn generates highly reactive species. If these conditions are not met, the efficiency of photosensitization will decrease at higher intensities when the number of absorbed photons per pulse approaches the number of dye molecules in the irradiated volume (ground state depletion).
The purpose of the present work is to investigate the implications of two earlier observations from work carried out at the Wellman laboratories [1], [2]. In 1994 we reported that two-color two-photon excitation of rose bengal could be used as an efficient oxygen independent mechanism to cause photosensitization. Photosensitization in the absence of oxygen was demonstrated in the killing of P388D1 cells grown in culture and in the inhibition of the enzyme acetylcholinesterase.
It was postulated that using nanosecond, high intensity, 532 nm excitation a similar mechanism should operate since only the triplet state will absorb when the ground state is completely depleted. However later experiments [2], under these conditions found that the inhibition of the enzyme could be explained by a purely singlet oxygen mechanism.
The critical difference between these two pieces of work is that the sequential, two color two photon work uses 532 + 640 nm light; the second photon is not absorbed by the ground state. Using single wavelength excitation, sequential two photon chemistry occurs in a region of ground state absorption. At high laser intensities all of the ground state should have been converted to excited triplet state and subsequent photochemistry should occur from the triplet manifold.
It is not clear why a Type I mechanism was not observed for the high intensity work and the results suggest a difference in the photochemistry of rose bengal when excited by these two methods. This work describes a series of experiments to investigate the photophysics of rose bengal when excited by two photons as a function of the wavelength of the second photon.
![]()
Materials and methods
Materials. Rose Bengal was obtained from Aldrich (Milwaukee, WI) with a stated purity of 97% and was used as received. All other chemicals for the buffer solutions were obtained from Sigma (St. Louis, MO).
Two photon laser flash photolysis. The two photon laser flash photolysis apparatus has been reported previously [3]. Briefly the second laser pulse is derived from a tunable optical parametric oscillator (OPO) pumped by a Nd:YAG (Spectra-Physics, Mountain View, CA). Synchronization of the OPO with the first excitation pulse (Nd:YAG, Continuum, Santa Clara, CA) was achieved using a Stanford Research Systems (Sunnyvale, CA) digital delay generator. Care was taken to ensure good spatial overlap of the two beams at the sample and for some experiments the laser beams were expanded to achieve this. The cuvette was sealed with a rubber septum and nitrogen saturated solutions were introduced into the cuvette via capillary tubing and a hypodermic needle. The cuvette was masked so that the monitoring beam only passed through the front 1 mm x 1 mm x 3 mm volume of the cuvette.
![]()
Results
Figure 1 shows the ground state absorption spectrum for rose bengal in phosphate buffer pH 7.
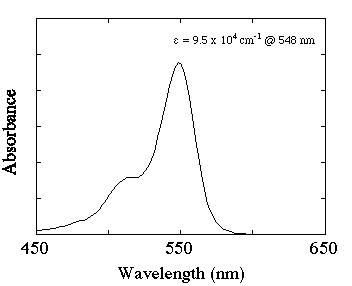
Figure 1. Ground state absorption spectrum of 5 ÁM rose bengal in 0.01 M phosphate buffer, pH 7
It can be seen from this diagram that when excited with two photons at 532 + 640 nm the second photon would not beabsorbed by the ground state. At 640 nm however there is triplet-triplet absorption and the second photon is absorbed only by the triplet state. (At 640 nm there is a small absorption due to the semioxidized radical of rose bengal but no photochemistry was observed from the absorption of red light by this species).
Two photon laser flash photolysis of a 5 ÁM rose bengal solution was carried out as a function of wavelength for the second photon. Figure 2 shows the absorption transient observed at 620 nm following two laser flash photolysis using 532 +640 nm excitation.
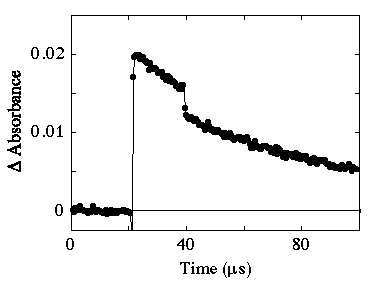
Figure 2. Transient observed at 620 nm following 532 + 640 nm excitation of rose bengal.
The delay in the arrival of the two laser pulses at the sample is approximately 20 Ás. It can be seen that the 640 nm light causes the absorption due to the triplet state to bleach. The mechanism by which bleaching occurs has been attributed to upper excited state photochemistry [1]. This regimen for two photon excitation has the advantage that the triplet state is selectively excited as there is no ground state absorption in this region.
The absorption transient observed following 532 + 532 nm excitation is shown in figure 3. It can be seen that the second laser pulse causes an increase in the triplet absorption. In this particular experiment it is likely that complete conversion had not been attained with the first pulse and hence there is an increase in the transient absorption seen with the second pulse due to trivial ground state absorption.
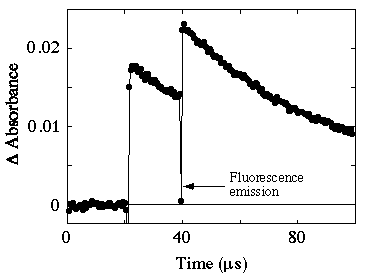
Figure 3. Transient observed at 620 nm following 532 + 532 nm excitation of rose bengal.
Under conditions where complete conversion is achieved with first laser pulse (using laser energies of up to 50 mJ/pulse) bleaching is not observed with a second 532 nm pulse. This experiment has also been carried out as a function of the delay time of the second pulse. It was not possible under any of the conditions used to observe bleaching with the second pulse. Figure 3 also shows fluorescence emission at the time of the second pulse. Using a gated intensifies diode array detector it was possible to obtain the emission spectrum of this fluorescence. It was found to be indistinguishable from ground state fluorescence.
Experiments were also carried out to investigate the bleaching as a function of wavelength of the second excitation pulse. The results of these experiment are shown in Figure 4.
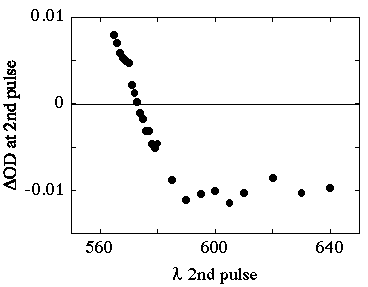
Figure 4. Bleaching of the transient absorption observed at 620 nm as a function of the wavelength of the second excitation pulse.
Above 580 nm the amount of bleaching with the second pulse is constant and wavelength independent. The amount of bleaching decreases with decreasing wavelength until at 570 nm there is no change in the transient absorption. At lower wavelengths an increase in transient absorption is seen with the arrival of the second pulse.
![]()
Discussion
The photophysics observed for the two photon excotation of rose bengal is summarized in Figure 5.
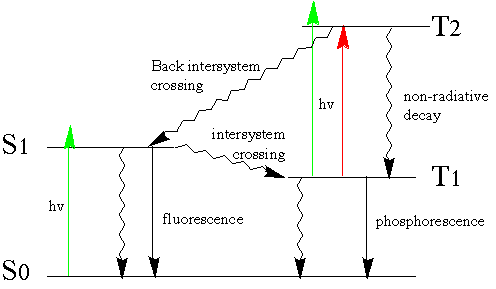
Figure 5. A modified Jablonski diagram for the two photon excitation of rose bengal.
The vertical green arrows represent sequential excitation with 532 nm laser light, the red arrow represents excitation with a 640 nm pulse. Excitation from T1 is achieved using the OPO laser. The most likely fate of a molecule excited into T2 is for it to undergo radiationless decay rapidly back to T1. T2 may also undergo upper excited state chemistry as postulated by Smith et al [1]. Alternatively it T2 may also ungo back intersystem crossing which is the the most likely mechanism by which fluorescence is observed at the time of the second pulse, irrespective of excitation wavelength.
Comparison of Figure 4 (bleaching of the transient absorption as a function of wavelength) with the ground state absorption spectrum strongly suggests that transient bleaching is the result of a balance between ground state and triplet-triplet absorption. This conclusion does not explain why bleaching was never observed in regions of ground state absorption. A number of different excitation geometries were set up to ensure overlap of the laser beams and homogeneity of the two lasers.
The results, summarized in Figure 4 can be interpreted in two ways. Either the photophysics of the first excited triplet state is strongly wavelength dependent or else there is some experimental artifact which masks the bleaching of the triplet state. If it was not possible to attain complete conversion for rose bengal, and the ground state absorption coefficient was large compared to that of the triplet, then a bleach may not be observed as in the 532 + 532 nm experiments described here. This would imply that the triplet-triplet absorption coefficient of rose bengal, determined by the complete depletion method, is lower than the true value. This possibility might be tested by redetermining the absorption coefficient for rose bengal using an energy transfer or comparative method. The interpretation that the photophysics changes dramatically with the wavelength of the second photon, is difficult to understand. The fact remains however, that under no experimental conditions were we able to observe bleaching with 532 + 532 nm excitation.
![]()
References
[1] Smith, G., W.G. McGimpsey, M.C. Lynch, I.E. Kochevar and R.W. Redmond (1994) An efficient oxygen independent two-photon photosensitization mechanism. Photochem. Photobiol. 59, 135-139.
[2] Lambert, C.R., H. Stiel, D. Leupold, M.C. Lynch and I.E. Kochevar (1996) Intensity-dependent enzyme photosensitization using 532 nm nanosecond laser pulses. Photochem. Photobiol. 63, 154-160.
[3] Redmond, R.W., I.E. Kochevar, M. Krieg, G. Smith and W.G. McGimpsey (1997) Excited state relaxation in cyanine dyes: A remarkably efficient reverse intersystem crossing from upper triplet levels. (1997) J. Phys. Chem. 101, 2773-2777.