DIFFERENT SENSITIVITY OF CELLS FROM TUMOR-BEARING ORGANISMS TO CONTINUOUS-WAVE AND PULSED LASER RADIATION (l =632.8 nm) EVALUATED BY CHEMILUMINESCENCE TEST
T.I. Karua, T.P.Ryabykhb, S.N.Antonovc, V.S. Letokhovd
aLaser Technology Research Center, Russian Academy of Sciences, 142092 Troitsk, Moscow Region; bN. Blokhin Cancer Research Center of Academy of Medical Sciences, Moscow; cInstitute of Radio Engineering and Electronics, Russian Academy of Sciences, Fryazino, Moscow Region; dInstitute of Spectroscopy, Russian Academy of Sciences, Troitsk, Moscow Region, Russian Federation.
Corresponding author Dr. Tiina Karu <karu@lls.isan.troitsk.ru>
ABSTRACT
Chemiluminescence test results were used to evaluate the sensitivity of human blood (healthy persons and colon cancer patients) and murine splenocytes (separated from intact animals and from mice with transplanted leukemia EL-4) to continuous-wave (CW) and pulsed He-Ne laser light (632.8 nm, 5x103 J/m2, 1-10 Hz, duty cycle 50 and 94%). It was demonstrated that CW radiation has in our experimental conditions practically no effect on the luminol-amplified chemiluminescence of four models under study. The pulsed radiation had a week inhibiting effect on the samples from healthy organisms but inhibited markedly the chemiluminescence of samples from tumor-bearing organisms. The effect depended on duration of dark period between pulses. A transient local heating mechanism was proposed to explain the inhibition of activity of NADPH-oxidase.
Key words: chemiluminescence, colon cancer, He-Ne laser, human blood, leukemia EL-4, murine splenocytes, transient local heating of absorbing chromophores.
1.INTRODUCTION
One of the methods used to evaluate changes in the oxidative metabolism of cells and tissues is the measurement of chemiluminescence (CL) associated with the generation of reactive oxygen species (ROS)1. Intact phagocytic and nonphagocytic cells have been known to feature spontaneous chemiluminescence (SCL) reflecting the original status of metabolic processes 2,1. When a cell is subjected to various stimuli, its chemiluminescence response may change, i.e., the amount of ROS generated by the cell may increase or decrease2.
We studied the effect of He-Ne laser radiation3 and semi-conductor laser radiation at 660-950 nm4 on the chemiluminescence of murine splenocytes and demonstrated that the effect of radiation on the SCL of the isolated splenocytes depended not only on the laser irradiation parameters (radiation dose, wavelength, pulse repetition frequency), but also on the physiological condition of the donor organism. While the effect of radiation of the SCL on normally functioning cells was not manifest, the response of cells from organisms suffering from pathological conditions was evident4 .
In the present work we investigated the effect of continuous-wave (CW and pulsed radiation of He-Ne laser on the CL of murine splenocytes (intact mice and the mice with transplanted leukemia EL-4) and human blood (healthy persons and patients with colon cancer). The aim of this work was double. First, we investigated the difference in CL responses of splenocytes and blood to laser radiation with changes in the health of the organism. Secondly, we compared the action of CW and pulsed radiation of the same wavelength and dose.
2. MATERIALS AND METHODS
Animals. (CBA + C57Bl) F1 male mice, 2 months old, were obtained from a local breeding colony and kept in standard conditions. Two groups of mice, 12 animals in each, were strictly selected by weight. The first, control group included intact mice, and the second, mice transplanted intraperitoneally with 5x105 EL-4 leukemia cells. The experiments with splenocytes were carried out within 5-10 days after the transplantation. In this period, the cellular composition of the spleen in the mice with transplanted leukemia differed only somewhat from that in the controls: there was observed an increased number of neutrophils and monocytes (Table 1) but the level of spontaneous CL was not elevated.
The mice were killed by cervical dislocation always at the same time of day near 12 A.M.- 1.P.M. to avoid circadian fluctuations in the activity of phagocytic cells5.
Table 1 Percentage concentration of various cells in the spleen of control mice and that of mice with transplated EL–4 leukemia cells (within 5–10 days after the transplantation)
Cells Mice group |
Lympho- cytes |
Mono- cytes |
Neutro-phils | Myelo- cytes |
Eosino- phils |
Plasma- cytes |
Others |
| Control | 87.0–95.0 | 0.2–0.6 | 1.0–4.6 | 0.2–4.8 | 0.4–2.0 | 0.2–0.8 | 0.4–2.6 |
| Leukemic | 84.0–87.6 | 0.4–1.6 | 4.8–9.0 | 2.0–4.8 | 0.4–3.2 | 0.4–1.0 | 1.4–2.6 |
Spleen cells. A suspension of spleen cells was prepared by gentle homogenizing of the spleen in a glass Potter homogenizer at 0° C in 5 ml of medium 199. The cells were centrifuged at 200g for 10 min, then resuspended in an ice bath in 0.83% NH4Cl in 0.05M Tris buffer pH = 7.4 (10 ml solution per 108 cells), and incubated in this solution for 5 min an ice bath. The cells were again centrifuged at 200g for 10 min and resuspended in 4 ml of medium 199 supplemented with 10% calf embryo serum. Counts of viable nucleated cells were performed with a hemocytometer, and suspension of 4x106 cells/ml was prepared for irradiation. In parallel with every irradiation experiment, smears for determining the cellular composition were prepared. The smears were fixed with methyl alcohol and stained with azureosin. One thousand cells were identified in every smear.
Every series of experiments included a control experiment with Candida albicans (object of phagocytosis) as described in paper3. These experiments served as a quality control of the cellular suspension subjected to irradiation.
Blood. Subject to investigation was a group of six patients (5 women and 1 men, 45-67 years of age) with cancer of the colon (stages IIIA-IV B) admitted to the clinic of the N. Blokhin Cancer Research Center of the Academy of Medical Sciences. Blood samples were taken from the cubital vein at 9-10 A.M. 200m l of heparinized blood was diluted in the proportion one part of blood to ten parts of medium 199. The blood samples taken from each donor were tested for spontaneous chemiluminescence (SCL) and CL induced by adding of Candida albicans as an object of phagocytosis (as described3) and for CL after irradiation as described below. The blood of the control group (6 clinically healthy persons) was treateded in the same way. The leukocyte count and blood-picture were determined for each sample.
Irradiation. The irradiation source was a He-Ne laser with an acoustooptical light modulator and electronic frequency synthesizer (Model Luch-1, Special Desing Department of the Institute of Radio Engineering and Electronics, Russian Academy of Sciences, Moscow Region, Fryazino, Russia). The samples were irradiated under either continuous-wave or pulsed conditions with a pulse repetition rate from 1 to 100 Hz at a duty cycle of 50 and 94%. The samples were placed in special round glass cells 110 m l in volume and irradiated from above through a fiber as described in detail in paper6. The irradiation scheme is presented in Fig.1. The exposure dose in all cases amounted to 5x103 J/m2, which corresponded to the range of optimal exposure doses producing the maximum effect for the given biological models10,12.
Fig.1.Scheme of irradiation procedure.
The output power of the CW beam at the distal end of fiber was 5 mW, the exposed area 0.28 cm2 , and the exposure time 28 s (CW mode), 30 s (pulsed mode with a duty cycle of 94%), or 56 s (pulsed mode with a duty cycle of 50%). Irradiation (as well as CL measurements) were performed in dark at room temperature. The irradiation parameters are summarized in Table 2.
Table 2. Parameters of pulsed He-Ne laser radiation used in our experiments.
| f, Hz | Duty T, s |
cycle t pulse,, ms |
94 %* t dark, ms |
Duty T, s |
cycle t pulse,, ms |
50%** t dark, ms |
||||
| 100 | 0.01 |
9.4 |
0.6 |
0.01 |
5.0 |
5.0 |
||||
| 50 | 0.02 |
18.8 |
1.2 |
0.02 |
|
10 | ||||
| 10 | 0.1 | 94 | 6.0 |
0.1 |
|
50 | ||||
| 1 | 1 | 940 | 60 |
1 |
|
500 | ||||
*Output power p=4.7 mW, I=169 W/m2, D=5´ 103 J/m2, irradiation time 30 s.
**Output power p=2.5 mW, I=89.3 W/m2, D=5´ 103 J/m2, irradiation time 56 s.
Chemiluminescence measurements. Immediately after the irradiation, the irradiated and control samples were transferred to photon counter tubes, containing 100 m l of luminol and 100 m l medium 199. Luminol (Serva, Germany) was used as 1 mM solution in Na phosphate- buffered saline, pH 7.2. As a photon counter, the chemiluminometer Model CL-3608 (Dialog, Moscow, Russia) connected with a personal computer, was used. The CL counts were measured during 10s for every time point indicated in kinetic curves. The graphical and statistical processing of the data obtained were carried out by means of the QPRO and STATGRAPHICS commercial program packages.
3. results
3.1 Comparison of responces of murine splenocytes: intact mice and mice with transplanted leukemia EL-4
The analysis of the kinetic SCL curves for the splenocytes of both groups of mice showed that they were practically the same within the measurement error. An example of such curves is presented in Fig. 2A (curve 1). The effect of laser radiation, if any, was always inhibitive as regards the SCL of the cells. Some original kinetic CL curves for the irradiated samples are presented in.Fig. 2A (curves 2-4) and the data expressed as the presentage of the SCL, in Fig. 2B. Below, only the per cent ratio between the maxima (25-30 min after exposure) of the corresponding curves for the treated and control samples are shown for quantitative evaluation of the irradiation effect.
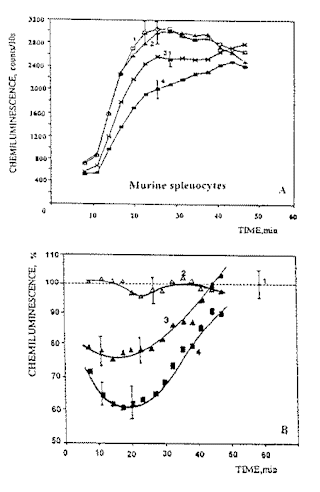
Fig.2.Examples of luminol-dependent chemiluminescence curves for spleen cells from mice transplanted with EL-4 leukemia cells: (1) spontaneous chemiluminescence; (2) chemiluminescence of cells exposed to CW radiation; (3) and (4) chemiluminescence of cells exposed to pulsed radiation with a duty cycle of 94% and 50% respectively. Radiation dose 5 x 103 J/m2 , pulse repetition frequency 1 Hz: (A) original recordings, and (B), per cent from SCL.
The first conclusion from the analysis of the results obtained is as follows. Exposure to CW laser light has practically no effect on the chemiluminescence of splenocytes in both the intact (control) and leukemic mice: 104.5 + 3% ,and 105 + 4.5%, respectively. The results of exposure to CW laser light are depicted by the shaded area in Fig. 3A. Exposing the spleen cells of both healthy and leukemic mice to pulsed laser radiation inhibited their chemiluminescence but in different degree. Also, the inhibition effect depended on the pulse repetition rate and duty cycle. first, radiation with a duty cycle of 50% inhibited CL more effectively than that with a duty cycle of 94%. Secondly, the CL inhibition effect in both cases depended on the pulse repetition frequency; it was a maximum (65-77% of the control level) at a pulse repetition frequency of 1 Hz and decreased as the rate was raised. Thirdly, attention is drawn to the fact that the splenocytes of the leukemic mice (Fig.3, curves 3 and 4) proved more sensitive to pulsed laser radiation than those in the intact mice.
![]()
Fig.3. Irradiation effect (inhibition of spontaneous luminol-dependent chemiluminescence) as a function of pulse repetition frequency:
A - (1) and (3) effect of radiation with a duty cycle of 94% on splenocytes from intact (control) mice and mice transplated with EL-4 leukemia, respectively; (2) and (4) effect of radiation with a duty cycle of 50% on splenocytes from control mice and leukemic mice, respectively. The effect of CW laser light on the spleen cells from both groups of mice (104.5± 4.5%) is practically the same as their spontaneous chemiluminescence (100 ± 5%) and is indicated by the shaded area.
B -effect of pulsed radiation with a duty cycle of 94% on blood from (1) healthy donors and (3) CC patients, and effect of pulsed laser radiation with a duty cycle of 50% on blood from (2) healthy donors and (4) CC patients. The chemiluminescence of blood in both healthy donors (98.3± 3.5%) and CC patients (100.4± 4.2%) following the exposure to CW laser light practically does not differ from spontaneous chemiluminescence (100± 5%) and is indicated by the shaded area.
Based on the data obtained (Fig.3A) one can delimit the region of parameters of pulsed laser radiation with a wavelength of l =632.8 nm where the effect of inhibition of the oxidative metabolism of splenocytes in the leukemia-transplanted mice was observed: pulse repetition frequency 1-50 Hz, duty factor 50%, and pulse duration 10-500 ms.
Thus, the results of the present study show that, first, the radiation sensitivity of cells differs between CW and pulsed radiation, the radiation wavelength and dose being the same. The effect of pulsed radiation depends on the pulse repetition frequency and duty cycle. Secondly, the sensitivity of cells in an organism with a tumor to pulsed laser radiation is higher than that of their conterparts in a healthy organism. this result supports our previous observations that cells in diseased organisms or cells cultivated under conditions other than optimal are more sensitive to laser radiation than their conterparts in healthy organisms or grown under optimal cultivation conditions7,15,17.
3.2. Comparison of responses of human blood: healthy persons and patients with colon cancer
The analysis of the blood SCL curves for healthy donors showed these curves to have a bimodal character: The first maximum was observed within 25 -30 minutes of the start of CL recording, and the second, more pronounced maximum occurred within 90-120 minutes. It should be noted that the ratio between the second and the first peak was always in the range 2-2.5. An example of these curves is presented in Figure 4 (curve 1).
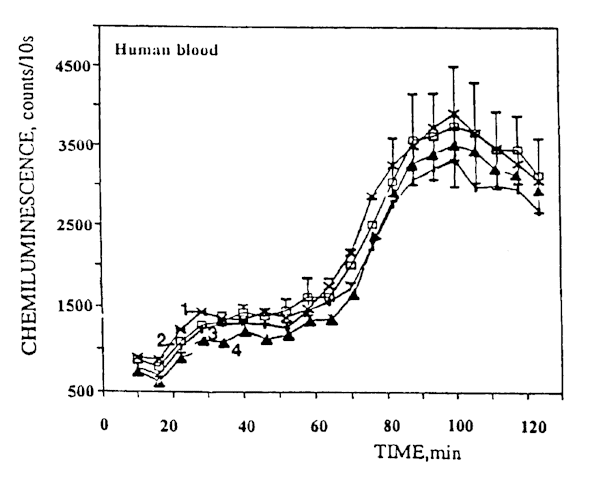
Fig.4. Examples of luminol-amplified blood chemiluminescence kinetic curves for healthy donors: (1) spontaneous chemiluminescence; (2) chemiluminescence following irradiation with CW laser light; (3) and (4) chemiluminescence following irradiation with pulsed laser light at a pulse repetition frequency of 1 Hz and a duty cycle of 50% and 94%, respectively.
The character of the blood SCL curves for patients with cancer of the colon (CC) differed from that of the control curves: the first peak (25-30 min after the start of CL recording) rose greatly (5-10 times). A typical example of such curves is presented in Figure 5 (curve 1). The ratio between the second and the first peak was around 0.5. Thus, the blood SCL curves for the CC patients differed from the corresponding curves for the healthy donors both qualitatively and quantitatively. Based on the literature data1, it may be assumed that the first peak is associated with the activity of neutrophils and the second, with that of macrophages. There being no sharp increase in the number of neutrophils in the blood samples from the CC patients in comparison with that in the controls, the increase of SCL may apparently be interpreted as the result of sensitization with respect to tumor cells. This hypothesis is supported by the fact that SCL per neutrophil in the patients with cancer of the colon was greater than that in the healthy donors. Similar results were obtained earlier when the blood of patients with tumors of other locations was analyzed8,9.
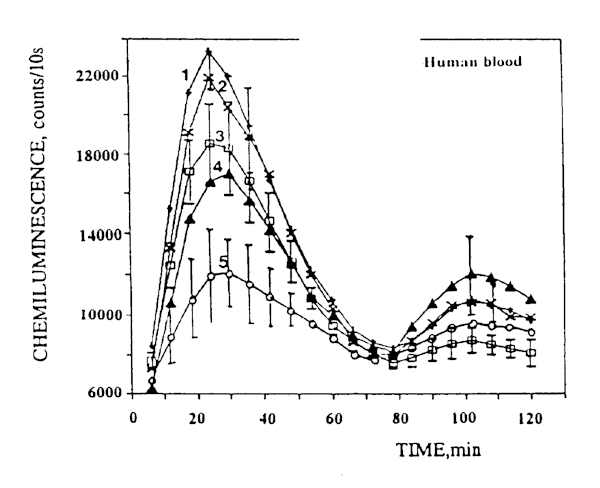
Fig.5. Examples of luminol- amplified blood chemiluminescence kinetic curves for patients with cancer of the colon: (1) spontaneous chemiluminescence; (2) chemiluminescence following irradiation with CW laser light; (3) and (4) chemiluminescence following irradiation with pulsed laser light at a pulse repetition frequency of 10 Hz and a duty cycle of 94%, and 50%, respectively; (5) chemiluminescence following irradiation with pulsed laser light at a pulse repetition frequency of 1 Hz and a duty cycle of 50%.
Quantitatively to evaluate the effect of irradiation, we next compared between the percent change of CL at the maximum of the kinetic curves (25-30 min after the start of measurement). An analysis of the CL curves for the irradiated blood samples allowed us to conclude that CW He-Ne laser radiation under our experimental conditions had no statistically significant effect on the CL of blood in both of the healthy donors and CC patients (curves 2 in Figs. 4 and 5 as typical examples ); the effect amounted to
98.3 ± 4.8%, and 100.4 ± 4.8%, respectively. The results of exposure to CW laser light are depicted by the shaded area in Fig. 3B.
In contrast to irradiation with CW light, exposing the blood samples from the CC patients to pulsed laser radiation inhibited their SCL. Fig. 5 presents some typical examples (curves 3 and 4). The effect depended on the pulse repetition frequency and duty cycle (Fig. 3B). At the same time, the effect of pulsed radiation on the CL of blood in healthy donors proved statistically insignificant in the overwhelming majority of cases. Some examples of kinetic curves are presented in Fig. 4 (curves 3 and 4) and the dependences on pulse repetition rate, in Fig. 3B (curves 1 and 2).
So, the effect on the blood samples from the CC patients depended, in contrast to those from healthy donors, both on the pulse repetition frequency (the reduction of the effect with the increasing pulse repetition frequency) and the duty cycle (Fig.3B). Radiation with the duty cycle of 50% inhibited SCL more effectively, especially at pulse repetition frequencies of 1-10 Hz. To illustrate, at pulse repetition frequency of 1 Hz and duty cycle of 50%, SCL is inhibited by 45%. As the pulse repetition frequency was raised, the effect decreased. One can delimit the region of effective action of pulsed laser radiation at l = 632.8 nm on the SCL of the blood in the CC patients under our experimental conditions: pulse repetition frequency 1-10Hz at a duty cycle of 50% or pulse repetition frequency of 1 Hz at a duty cycle of 94%.
Thus, the results of this investigation based on the evaluation of the ability of whole blood cells to generate highly toxic oxygen radicals show, first, that they are much more sensitive to pulsed than CW laser radiation (the radiation wavelength and dose being the same). Secondly, the sensitivity of blood in the patients suffering from cancer of the colon to pulsed laser radiation is enhanced in comparison with that of blood in the healthy donors.
3.3. Effect of dark period between pulses
Figure 6 presents the dependences of irradiation effect (inhibition of luminol-amplified chemiluminescence evaluated by CL kinetic curves 25-30 min after the irradiation) on the pulse duration (for radiation parameters see Table 2) for splenocytes of intact and leukemic mice (parts A and B, respectively) as well as for blood of healthy persons and cancer patients (parts C and D, respectively). As seen in Fig. 6 B,D, the CL of splenocytes of leukemic mice as well as that of the blood of cancer patients is strongly inhibited by the radiation with duty cycle of 50%. The inhibitive action is less pronounced by the radiation with duty cycle of 94%. In all cases there exists also a dependence of the effect on the pulse duration (the effect increases as the pulse duration is increased).
It can be seen by comparison of Figures 6A,C with Figures 6B, D, that the dependences of irradiation effect on pulse duration for splenocytes of intact mice and blood of healthy persons are much less pronounced as compared with respective data for ill organisms. In other words, the models considering healthy organisms appeared to be less sensitive to the pulsed radiation at 632.8 nm compared to the splenocytes from leukemic mice and blood of cancer patients.
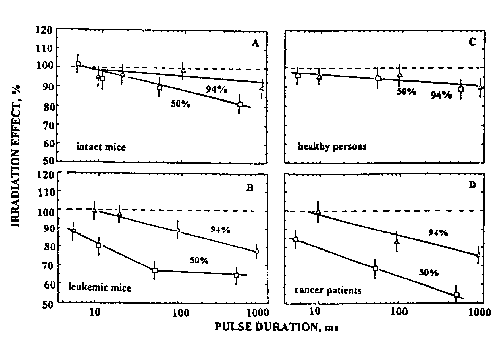
Fig. 6. Effects of pulsed He-Ne laser radiation (D=5 x 103 J/m2, duty cycle 50 or 94%) on luminol-amplified chemiluminescence of splenocytes from (A) intact and (B) transplanted with leukemia EL-4 mice, and blood of (C) healthy persons and (D) patients with colon cancer as dependence on pulse duration.
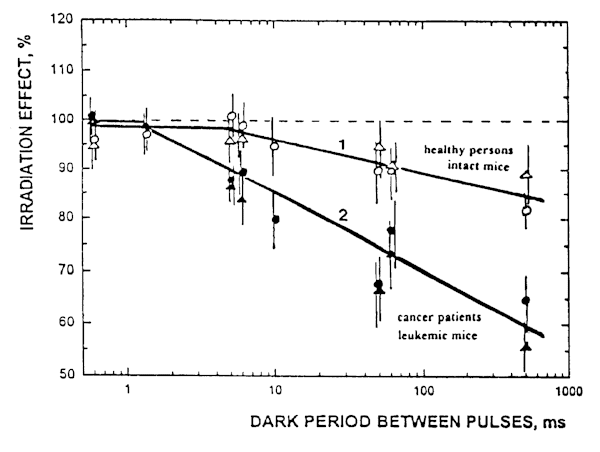
Fig. 7. Effect of of pulsed He-Ne laser radiation upon luminol-amplified chemiluminescence of (1) splenocytes (o ) and blood (D ) of healthy organisms, and (2) splenocytes (l ) and blood (black D ) of tumor-bearing organisms as a dependence on dark period between the pulses.
Figure 7 presents the dependence of irradiation effect on the dark period between pulses (the data for radiation parameters can be found in Table 2). As it is seen in Figure 5, with this parameter all data for inhibition of CL by pulsed radiation can be presented by two curves: one of them is related to healthy persons and intact mice (curve 1) and another one, to cancer patients and leukemic mice (curve 2).
It is possible to define a region of values of dark periods between pulses where there exists a statistically significant difference between curves 1 and 2 (p< 0.05): the effect starts at dark period between pulses of 10 ms and increases with increasing the dark period (500 ms in conditions of our experiments). An attention is drawn to the fact that the cells from tumor-bearing organisms proved to be more sensitive to pulsed laser radiation than those of the healthy ones. For example, at the value of dark period between pulses of 500 ms, the CL of samples from healthy organisms is suppressed only by 15% (which value is statistically insignificant as compared to spontaneous chemiluminescence (SCL) of nontreated samples, 100%). The radiation with the same parameters suppresses the CL of samples from tumor-bearing organisms approximately by 40%, which value is statistically significant difference both from the value of SCL as well as from the value of CL of irradiated samples from the healthy organisms.
4. DISCUSSION
The luminol-amplified chemiluminescence measurement was used in the present study as a test for evaluating sensitivity of four model systems (blood of healthy persons and cancer patients, splenocytes from intact mice and mice with transplanted leukemia EL-4) to pulsed He-Ne laser radiation (parameters of which are presented in Table 2).
The irradiated samples of both types, diluted blood and suspension of splenic cells, are complex mixtures of different types of cells. Not all of these cells are contributing in measured values of CL. The CL is proportional with the amount of ROS produced by phagocytic cells and in particular, it is proportional to the rate of H2O2 production, which is arising by spontaneous dismutation of superoxide anion O2 & ¯ 13. It is believed that the CL measurement reflects the activity of NADPH-oxidase1. NADPH-oxidase is an electron transport chain in cellular membrane found in all phagocytic cells (e.g. neutrophils, monocytes, macrophages) and recently also in some nonphagocytic cells like B and T lymphocytes and fibroblasts11. The NADPH-oxidase contains a flavocytochrome b and uses NADPH as an electron donor to reduce oxygen to O2 & ¯ 13. Its activity is low in intact cells but can be increased rapidly as a response to different stimuli1. In other words, the kinetics of chemiluminescence responses reflects the process of NADPH-oxidase activation in its early steps.
It was found in the present study that the crucial parameter of pulsed He-Ne laser radiation for inhibition of luminol-amplified CL is the duration of the dark period between pulses. Secondly, it appeared that the samples from tumor-bearing organisms appeared to be more sensitive to this type of radiation as compared with the samples from healthy organisms (the CL was inhibited by an greater degree Fig.7). One should note that the tumor cells themselves as well as the cells from tumor-target organs were not the objects of investigation in the present study. Also one should emphasize that the reason for the enhanced sensitivity of cells from tumor-bearing organisms is still to be understood. An explanation of this question is out of frames of the present investigation.
Why does the effect of inhibition of NADPH-oxidase activity occur only under pulsed irradiation conditions and only on the condition that the dark period lasts at least some milliseconds (Fig. 7)? In principle one should consider the possible mechanisms responsible for this effect at all organization levels (physical, chemical, biological), as well as at both the molecular and macroscopic levels. Let us consider here the physical point of view.
The radiation-exposed object is a suspension of cells with an average diameter of D » 15-20 m m18. We exclude from the consideration the cells which are not responsible for chemiluminescence as for example the red blood cells and platelets. The irradiated cells are spaced at an average center-to-center distance of apart, which is determined by the concentration of the cells in the solution, N=1/L3. In our case, N=4x106 cells/ml, which corresponds to L» 70 m m. Thus, the average distance between the membranes of the adjacent cells is (L - d) » 50 m m, and the ratio of the total volume of the cells to the volume of the solvent is given by the dilution factor
![]() 3
(1)
3
(1)
In our case, d » 1 %.
Under irradiation, the absorbing components (the chromophores) of the exposed cells convert within a very short period of time (less than 1 ns) a sizeable proportion, h ò of the absorbed energy to heat in a radiationless manner. Note that the absorbing chromophores is our case are not establishod so far, but for example, the NADPH-oxidase contains the flavine moiety which semiquinone form can absorb the light at l = 632.8 nm19. As a result, there takes place a short-term heating of the absorbing microregions by a small amount of D T 14.Thanks to heat diffusion heat propagates first throughout the bulk of the cells and then throughout the intercellular space (solvent), and thermal equilibrium is thus reached.
The time it takes for heat to propagate throughout the bulk of the quasispherical cell is
![]() (2)
(2)
where c is the thermal diffusivity of the intercellular medium. Under our experimental conditions, D=20 m m and c = 1.3 x 10 -3 cm 2/s, and so tcell » 0.1 ms. Thereafter heat starts diffusing the solution, so that during the time
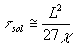 (3)
(3)
thermal equilibrium is reached in the solution and temperature gradients vanish there. In our conditions, tsol » 1ms.
Thus, there are three characteristic times describing the effect of local transient heating, namely, (1) the heating time of the light-absorbing microregions (cells), governed by the laser pulse duration tp; (2) the time tcell it takes for heat to diffuse beyond the heated microregion of diameter D, which may be longer or shorter than tp; and (3) the time tsol it takes for heat to diffuse for a distance comparable with the average distance L between the microregions, in the course of which there takes place the equalization of the spatial heating microirregularities. If the condition L >> D is satisfied, these times satisfy the relation
tsol >> tcell. (4)
It should be emphasized that temperature gradients arise in the solution as a result of the temporally nonstationary (transient) and spatially nonuniform distribution of the absorbed light energy in conditions of pulsed irradiation with a pulse duration of
tp < tsol. · (5)
In the case of continuous-wave irradiation or spatially uniform light absorption, no such effects occur.
With this hierarchy of characteristic times in mind, one can single out the following modes which are qualitatively illustrated in Fig. 8 and which comply with the conditions of the experiments described above.
In the experiments, the laser pulse duration was in the range 5´ 10-3 s < tp < 1 s, i.e., the time tp was always much longer than the time tcell. For this reason, in the course of the laser pulse there first took place during the short time tcell a local heating by an amount
![]() (6)
(6)
of where k cell is the light absorption coefficient (averaged over the cell volume), I is the radiation intensity (W/cm2), and c is the specific heat capacity of the cell. Under our experimental conditions, the absorptivity of the cellular solution is c0 @ 1 cm-1. Since the solvent (water) practically absorbs no radiation at l = 632.8 nm, the absorptivity of the intercellular medium (averaged over its volume) is k cell = k 0/d @ 102 cm-1, where d is the dilution factor of the cellular solution (d = 10-2 in our conditions). At a radiation intensity of I = 10-20 mW/cm2, the temperature rise DT0 @ (2.5 to 5)´ 10-5 ° C. Thereafter there takes place a gradual heating during the course of the laser pulse in conditions of heat diffusion from the heated microregions as given by the equilibrium (7) and illustrated in Fig 8A.
DT(t) = DT0(t/tcell)1/2, t £ tp, tsol. (7)
Depending on the laser pulse duration, heating in the course of the pulse may be as high as
2´ 10-4-5´ 10-3 ° C.
After the laser pulse comes to an end, there occurs the cooling of the microregions as a result of diffusion of heat into the intercellular space during the time tsol within which temperature gets equalized over the whole volume. This is possible if the dark period td between the successive laser pulses satisfies the condition
td ³ tsol. (8)
In our experimental conditions, this corresponds to a pulse period of td ³ 10-3 s. In that case, the heating modulation amplitude DT reaches its maximum, the average heating being negligible: Dtav @ dDT @ 10-4-10-6 ° C.
Otherwise when the interval between the successive laser pulses is short, the cooling dT << DT, the heating modulation amplitude drops, and there occurs an averaged heating of the entire exposed microregion (Fig. 8B), which is equivalent to heating in conditions of continuous-wave irradiation.
All the above estimates of the temporal effects are in qualitative agreement with the experimental data presented in Figs. 6 and 7. The slow rise of the effect with the increasing laser pulse duration (Fig. 8) is associated (naturally, not necessarily directly) with the increase in the pulsed heating amplitude DT, and the explicit dependence of the effect on the length of the dark period (Fig. 7) is due to the rise of the periodic heating modulation amplitude dT.
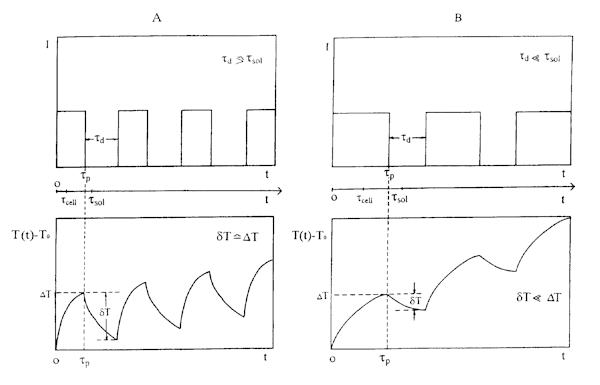
Fig. 8. Time dependences (below) of temperature rise T(t)-T0 due to transient local heating of absorbing microregions in solution (tcell is the heat diffusion time from a local microregion, tsol is the time of heat diffusion between neighbour microregions) for (top) various time dependences on the intensity of laser pulses: a) td ³ tsol; b) td < < tsol;
Based on these notions, one can formulate optimal experimental conditions wherein the effect of impulse modulation of local heating at the cellular level should be a maximum. First, the radiation pulse duration should satisfy the condition
tp £ tcell @ 10-4 s, (9)
and the radiation intensity, I ³ 1-10 W/cm2, which provides for DT0 @ (2.5 to 25)´ 10-3 ° C. The interval between the successive pulses should be, for example, td > 0.1 s, i.e., the average radiation intensity will be a mere 1-10 mW/cm2. Note that if condition (9) is satisfied, the temperature gradient
![]() (10)
(10)
will reach its maximum which may be as high as 102 ° C/cm. The high temperature gradient may also be responsible for the effects observed.
So, in addition to the photochemical mechanism suggested earlier7,17 for the low-intensity laser effects, it is expedient also to consider the photothermal mechanism based on the local transient heating of real spectrally and spatially heterogeneous bioobjects. Of course, these mechanisms are not alternative and can coexist quite well17 . However, what is most difficult at this point is to explain the manifestation of the photothermal mechanism in biochemical processes. One can put forward the following qualitative considerations in this respect.
During the course of the pulsed heating of a cell and its membrane by a part of degree centigrade there may take place changes in the characteristics of both the membrane itself (its elasticity, shape, etc.) and the enzymes taking part in this particular metabolic pathway and incorporated therein. This effect can occur only if the laser pulse-repetition period D t ³ tsol = 1ms. In that case, by the instant the next laser pulse arrives, a nonuniform temperature distribution in the bulk of the solution relaxes completely, causing but an insignificant average heating by an amount of d T » 10-5 0C. Such a transient local heating mechanism can explain the effect of inhibition of oxidative metabolism observed to occur in phagocytic cells, provided that the pulsed heating of the membrane by a few degrees centigrade does affect the characteristics of incorporated enzymes responsible for the respiratory burst, or, to be more exact, lowers somewhat their activity. Inasmuch as the activity of NADPH-oxidase depends on its flavin cofactor16, one can assume that the suppression of CL by pulsed He-Ne laser radiation could be connected with its inactivation.
There is a growing realization that NADPH-oxidase system is not confined to phagocytes. The CL accompanies also such a physiological reactions like proliferation, blasttransformation, chemotaxis, receptor-ligand coupling1. So, the possibility to inhibit NADPH-oxidase activity by pulsed He-Ne laser radiation might have a larger interest for other model systems as well.
The next question of interest is, why does the CL inhibition effect manifest itself more in cases of cells from diseased organisms that in cases of cells from healthy ones. First of all, it should be emphasized that the medical aspects of the problem were not under study here. The blood as well as the splenocytes from ill organisms were used as model systems. And what more, the tumor cells (and the cells from tumor target organs) were not used in this study.
The shape of kinetic curves as well as absolute values of SCL and induced by some phagocytic stimulus CL of blood differ in case of healthy persons and cancer patients. Attempts have been made to use this circumstance for diagnostics8,9,20-24 but until now no strict and reliable method does not exist. Also the CL of blood plasma has been under study for comparative diagnostics of cancer25,26. In this case, the CL originates from lipid hydroperoxides. These measurements also did not indicate the strict value of CL measurement.
An attempt was also made to find differences in blood CL responses of healthy persons and cancer patients to CW He-Ne laser radiation24. CW He-Ne laser radiation was found to activate SCL and modulate stimulated by an ionophore CL both in healthy blood and blood of patients with gastric cancer. The authors interpreted their results in frames of priming action of laser radiation. There was no clear difference in responses of blood of two experimental groups as well as between responses of separated from blood of these two groups24.
5. CONCLUSION
As a result of our experiments we found by CL test a statistically significant clear difference in reaction of samples from healthy and tumor-bearing organisms to irradiation with pulsed light. The difference depended on light parameters, decisive armong them appeared to be the duration of dark period between the pulses (Figure 7). This regularity was the same in both models under study (human blood and murine splenocytes). Quite possible, that this finding can be used for development of a method of cancer detectio in the future.
A transient local heating mechanism explaining the suppressive action of radiation (probably, an inactivation of NADPH-oxidase) was proposed.
At least two questions are still open. First, is that regularity specific for tumor-bearing organisms or it is valid in case of other diseases (e.g., inflammation), too. Second, why the inhibitive effect of pulsed radiation manifests itself more in case of cells from diseased organisms that in cases of cells from healthy ones. At the present stage of research one can only assume that the affected enzyme (NADPH-oxidase) incorporated in the plasma membrane recovers (kinetically or structurally) in one case better that in other. Anyway, the both issues require further research.
acknowledgments
Sandia National Laboratory (USA) and Ministery of Science of Russian Federation (project 8.1 in "Fundamental Spectroscopy") are acknowledged for financial support.
REFERENCES
1. Sbarra, A.J., and Strauss, R.R. (eds) (1988). The Respiratory Burst and Its Photobiological Significance. New York, London: Plenum Press.
2. DeSole, P. (1989) Polymorphonuclear chemiluminescence: Some clinical applications. J. Biol. Chemilum. 4, 251-262.
3. Karu, T.I., Ryabykh, T.P., Fedoseyeva, G.Ye., and Puchkova, N. I. (1989) He-Ne laser induced respiratory burst of phagocytic cells. Lasers Surg. Med. 9, 585-588.
4. Karu ,T.,Andreichuk, T., and Ryabykh, T. (1993). Changes in oxidative metabolism of murine spleen following laser and superluminous diode (660-950 nm) irradiation: effect of cellular composition and radiation parameters. Lasers Surg. Med. 13, 453-462.
5. Knuszinsky, A., and Fischer, H. (1981) Circadian fluctuations in the activity of phagocytic cells in blood, spleen, and peritoneal cavity of mice as measured by zymosan-induced chemiluminescence. J. Immunol. 127, 2508-2511.
6. Karu, T.I., Pyatibrat, L.V., Kalendo G.S., and Esenaliev, R.O. (1996) Effects of monochromatic low-intensity light and laser irradiation on adhesion of HeLa cells in vitro. Lasers Surg. Med. 18, 171-177.
7. Karu, T.I. (1989). Photobiological Fundamentals of Low-Power Laser Therapy. Chur, London: Harwood Acad. Publ.
8 Heberer, M., Ernst, M.,Durig,H., Allgower, M., and Fisher,H. (1982) Measurement of chemiluminescence in freshly drown human blood. II. Chemical application of zymosan-induced chemiluminescence. Klin. Wochenshr. 60, 1443-1448.
9. Trulson, A., Nilsson, S., Venge, P. (1989) Lucigenin-enhanced chemiluminescence in blood is increased in cancer. Am. J. Clin. Pathol. 91, 441-445.
10.. Karu, T.I.,Ryabykh, T.P. and Antonov, S.N.(1995). Different effects of countinous wave and pulsed laser radiation (l =632.8 nm) on oxydative metabolism of splenocytes. Doklady Akad. Nauk (Engl. transl.- Doklady Biophysics) 345, 407-409.
11. Segal, A.W. and Abo, A. (1993). The biochemical basis of the NADH-oxidase of phagocytes. Trends Biochem. Sci. 18, 43-47.
12. Karu, T. and Ryabykh, T. (1995) Effect of suppression of oxidative metabolism of cells with pulsed radiation of He-Ne laser depends on duration of dark period between pulses. Doklady Akad. Nauk (Engl. transl.- Doklady Biophysics) 353, 676-678.
13. Baggiolini, M. and Wyman, M.P. (1990) Turning on the respiratory burst. Trends Biochem. Sci. 15, 69-72.
14. Letokhov,V.S. (1991) Effects of transient local heating of spatially and spectrally heterogeneous biotissue by short laser pulses. Il Nuovo Cimento 13D, 939-948.
15. Karu, T.I., Andreichuk, T.N. and Ryabykh, T.P. (1995) On the action of semiconductor laser radiation (l =820 nm) on the chemiluminescence of blood of clinically healthy humans. Laser Life Sci.6, 277-282.
16. Tauber,A.I. (1982) The human neutrophil oxygen armory. Trends Biochem. Sci. 7, 411-414.
17. Karu, T. (1997). The Science of Low Power Laser Therapy. London: Harwood Acad. Publ.
18. Barne, R.M. and Levy,M.N., eds. (1988). Physiology, Sec. ed., St. Luis, Wasch. D.C., Toronto: The C.V.Mosby Comp., Ch. 24.
19. Ghisla, S. and Massey, V. (1989). Mechanisms of flavoprotein-catalyzed reactions. Eur. J. Biochem. 181, 1-17.
20. Hirano, T. (1984) Changes in polymorphonuclear leukocyte motility under agorose and luminol-dependent chemiluminescence response in patients with gastric cancer. Gastroenterol. Jpn.19, 447-456.
21. Korkina, L.G., Suslova, T.B., Samochatova, E.V., Cheremisina, Z.P. and Rumyantsev, A.G. (1985) Blood and bone marrow cell chemiluminescence in children with leukemias. Hematol. Transfus. 30, 24-28.
22. Bart, J., Amelsberg, A., Ravens, K.G., Petermann, W. (1987) Unterschiede in Aktivitä t und Stimulierbarkeit von Granulozyten und Monozyten bei Patienten mit Bronchialkarzinom und Normal-persones, gemessen in der luminol-abhä ngigen Chemiluminezenz im Vollblut. Prax. Klin. Pneumol. 41, 712-713.
23. Schepetkin, I.A., Cherdyntseva, N.U., Borunov,E.V., Skutina, I.L. and Naumov, S.A. (1991) Decreased luminol-dependent chemiluminescence response of neutrophils to recombinant human tumour necrosis factor in patients with gastric cancer. J. Cancer Res. Clin. Oncol. 117, 172-174.
24. Schepetkin, I.A., Udut,V.V. and Karpov,A.B. (1994) Chemiluminescence response of human neutrophils to He-Ne laser irradiation (in vivo and in vitro). J.de Physique IV, C4, 219-229.
25. Shpolyanskaya, A.M., Mitrofanov, A.I., Perelman, M.I., Bondarev, I.M. and Zhuravlev, A.T. (1976) Differences in biochemiluminescence intensity of blood serum in tuberculosis and lung cancer. Psychoenergetic Systems, 1, 203-204.
26. Chebotarev, G.E., Baraboi,V.A., Ganul, V.L., Dorfman, V.L., Orel, M.V. and Tatsii, J.A. (1979) Spontaneous blood serum chemiluminescence in surgical interventions experimentally and in patients with lung cancer. Onkologiya (Kiev), 14 , 64-68.