Relative contribution of apoptosis and random necrosis in tumour response to photodynamic therapy: effect of the chemical structure of Zn(II)-phthalocyanines
Giulio Jori and
Clara
Fabris
Department of Biology,
University of Padova, via U.
Bassi 58/B, 35131 Padova, Italy
Abstract
Zn(II)-phthalocyanine (ZnPc) and its octapentyl (ZnOPPc) and octadecyl (ZnODPc) derivatives were intravenously injected at a dose of 1.46 Ámol/kg to female Balb/c mice bearing an intramuscularly transplanted MS-2 fibrosarcoma. Pharmacokinetic studies showed that in all cases the maximal concentration of phthalocyanine in the tumour was reached at 24 h post-injection: the efficiency and selectivity of tumour targeting slightly increased upon increasing the length of the alkyl substituents. Irradiation of the neoplastic lesion (620-700 nm light, 180 mW/cm^2, 300 J/cm^2) at 24 h after photosensitizer administration induced a significant delay of tumour growth, which was largest (ca. 11 days) for ZnPc and smallest (ca. 3.5 days) for ZnODPc. Electron microscopy investigations of irradiated tumour specimens showed that ZnPc caused an early direct damage of malignant cells, largely via processes leading to random necrosis, although a limited contribution of apoptotic pathways was detected. The importance of apoptosis increased upon using ZnOPPc and especially ZnODPc as the photosensitizers possibly owing to a different partitioning in different compartments of cell membranes.
1. Introduction
Photodynamic therapy (PDT) of tumours has been known for over twenty years [1] and its usefulness for cancer control has been repeatedly demonstrated in both experimental models and clinical treatment of a variety of solid tumours [2]. However, in spite of intensive research, the information on the mechanisms controlling the response of malignant lesions to this phototherapeutic modality are still largely incomplete. This situation reflects, at least in part, the complex interplay among several different factors which affect the transport of the systemically injected photosensitizing agents to the tumour, hence their distribution among the diverse tissue and cell compartments [3]. In actual fact, it has been ascertained that the action of PDT can involve various subtissular targets, including the neoplastic cells, the blood vessels and the non-vascular stroma [4] to an extent which is strongly dependent on the local concentration of the photosensitizer. The overall picture became even more complex as a consequence of the recent observation [5] [6] that PDT-induced tumour damage can also occur via apoptotic pathways.
The localization pattern of photosensitizers and the correlation between these patterns and the primary targets of PDT are widely influenced by the physico-chemical structure of the drug, in particular the degree of hydrophobicity [3]; this parameter often determines the interaction of the photosensitizer molecules with serum proteins, as well as their partitioning in specific sites or domains of cell organelles [7]. Therefore, it appears important to further our knowledge on this topic in order to optimize the development of second generation photosensitizers with improved selectivity of tumour targeting and phototherapeutic efficacy.
In this paper, we describe our findings on the mode of PDT action by some derivatives of Zn(II)-phthalocyanine (ZnPc) using an animal model (the MS-2 fibrosarcoma intramuscularly implanted in Balb/c mice) which has been studied in detail in our laboratory. Phthalocyanines represent interesting substrates for studies on the relationships between chemical structure and phototherapeutic activity owing to the ready synthesis of metal-chelated, axially ligated or peripherally substituted derivatives [8] and their photobiological properties which are particularly suitable for PDT applications [9].
2. Materials and methods
ZnPc was a product of Ciba (Basel, Switzerland), while 1,4,8,11,15,18,22,25-octakis-pentyl (ZnODPc) and -octakis-decyl (ZnODPc)-phthalocyanines were prepared by chemical synthesis [10] and kindly supplied by Prof. D.A. Russell (University of East Anglia, UK). The chemical structure of the three phthalocyanines is shown in Fig. 1. Owing to the very low solubility in water, the phthalocyanines were i.v. injected after incorporation into suitable delivery vehicles, namely small unilamellar liposomes of DL-a-dipalmitoyl-phosphatidylcholine (DPPC, Sigma) in the case of ZnPc, and oil emulsions of Cremophor EL (Sigma) in the case of ZnODPc and ZnOPPc. The procedures adopted for the preparation and characterization of the injectable formulations have been described elsewhere [11] [12]. As an animal model, we used a MS-2 fibrosarcoma (originally obtained from Istituto Nazionale Tumori, Milano) intramuscularly injected into the right hind leg of female Balb/c mice, having a body weight of 20-22 g. The mice were injected into the tail vein with the phthalocyanines (1.46 mmol/kg) on the 6th-7th day after transplantion when the external diameter of the tumour reached 0.7-0.8 cm. No spontaneous remission or regression of the tumour was observed during our investigations.
![]()
Fig 1
Chemical structure of Zn-phthalocyanines
![]()
At predetermined times after injection of the phthalocyanines the mice were sacrificed by prolonged exposure to ether vapours, the tumour and selected tissues were quickly removed, washed twice with saline and the content of the photosensitizer was measured by spectrophotofluorimetric analysis after chemical extraction according to the procedure previously validated [13]. At the same time, the blood (ca. 1 ml) was taken intracardiacally, centrifuged for 15 min. at 3,000 rpm in order to remove the erythrocytes and the serum thus collected was diluted with 2% aqueous SDS and analyzed for the phthalocyanine concentration. At least three mice were independently analyzed at each time point.
In another set of experiments, groups of five mice were irradiated at 24 h after injection of the single phthalocyanines by using a quartz/halogen lamp (Teclas, Lugano, Switzerland) which was equipped with bandpass optical filters to isolate the 620-700 nm wavelength range. The lamp was operated at 180 mW/cm^2 for a total delivered light dose of 300 J/cm^2; the beam was piloted to the irradiation site by a bundle of optical fibers whose tip (diameter 0.8 cm) was kept at a distance of 1 cm from the tumour surface. Control studies showed that under these irradiation conditions but in the absence of photosensitizer there was no appreciable effect on the rate of tumour growth, while the tumour temperature does not rise beyond 38░C, so that any hyperthermal effect can be discounted.
At predetermined post-irradiation times, the mice were sacrificed, the tumour was excised, fixed with glutaraldehyde for ultrastructural studies, and the ultrathin sections were analyzed by an Hitachi H-600 electron microscope [14].
3. Results
Selected pharmacokinetic properties of ZnPc, ZnODPc and ZnOPPc are summarized in Table 1. In all cases, the largest recovery of the phthalocyanine from the neoplastic tissue was found at 24 h post-injection, in agreement with previous findings [14] [15] [16]. There appears to be a modest increase in both the efficiency and selectivity of tumour targeting upon increasing the length of the alkyl chains protruding from the tetraazaisoindole macrocycle. Moreover, the phthalocyanines were accumulated in large amounts in the liver, as it is typical of most hydrophobic photosensitizers, which are predominantly eliminated from the organism through the bile-gut pathway [17]. On the other hand, there were only minor differences in the rate of serum clearance, which is correlated with the persistence of cutaneous photosensitivity [15], [16].
![]()
Table 1 |
|||
| Parameter | ZnPc | ZnOPPc | ZnODPc |
| Max. tumour accumulation (24 h, nmoles/g) |
1.93 ▒ 0.21 | 2.42 ▒ 0.32 | 3.21 ▒ 0.16 |
| Tumour/muscle concentration ratio at 24 h | 3.97 | 5.06 | > 5.60 |
| Max liver accumulation (nmoles/g) |
3.27 ▒ 0.62 (24 h) |
7.95 ▒ 1.19 (48 h) |
10.16 ▒ 1.03 (24 h) |
| Serum half-life (h) | 9.3 | 7.3 | 6.8 |
![]()
On the basis of the similarity in the pharmacokinetic properties, identical PDT protocols were used for the three phthalocyanines. However, the type of tumour response to the irradiation was significantly different for the different photosensitizers. ZnPc-treated tumours developed a massive haemorrhagic necrosis within 24 h after the end of PDT, with gradual formation of eschar and a marked reduction in the rate of tumour growth: the time interval for the tumour growth to a volume of 0.3 cm^3 from the 0.04 cm^3 volume measured at the time of irradiation was 19.0 ▒ 2.0 days as compared with 8.2 ▒ 1.9 days for control unirradiated mice. This parameter was found to be 14.8 ▒ 2.3 days and 11.7 ▒ 1.8 days for ZnOPPc- and ZnODPc-photosensitized tumours, respectively. However, the irradiation of the MS-2 fibrosarcoma in the presence of the alkyl-substituted ZnPcs was not followed by the appearance of eschar or other necrotic features: in the case of ZnOPPc the main observable feature was the formation of an extensive oedema at the level of the irradiated area, while for ZnODPc a shrinkage of the tumour volume could be clearly detected within 24 - 48 h after PDT.
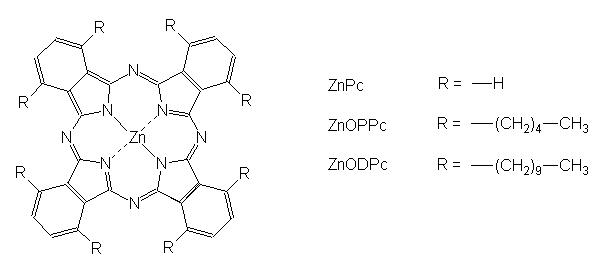
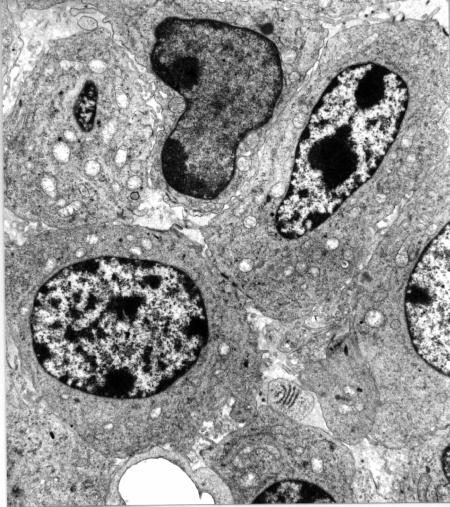
A clue to the interpretation of these apparently different patterns was provided by electron microscopy investigations. As shown in Fig. 2, ZnPc photoinduced an early direct damage of malignant cells, while other compartments of the tumour tissue were affected to a minor extent. This behaviour is typical of several hydrophobic porphyrin derivatives, which are preferentially transported by serum lipoproteins, and are specifically accumulated by malignant cells through a receptor-mediated endocytotic process [18]. The main features of the photodamaged cells were represented by swollen or optically empty mitochondria, a dilation of the cisternae of the endoplasmic reticulum, extensive cytoplasmic vacuolization and a partial detachment of the perinuclear membrane: this suggests that multiple subcellular sites were involved in the initial stages of the photoprocess, thereby eliciting random degenerative phenomena [4] , [19]; in a few cases, however, malignant cells showing typical features of apoptosis, such as deformation of the nucleus, chromatin condensation, floating of chromatin aggregates in the cytoplasm and formation of blebs on the plasma membrane could be detected (Fig. 3a) (Fig. 3b).
![]()
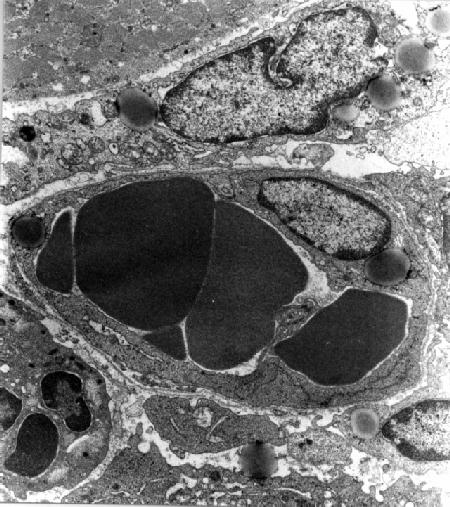
Fig 2
Tumor specimen obtained at 8 h after PDT with ZnPc (x 7,500). The endothelial cells are well preserved, while the damage of neoplastic cells is markedly pronounced with dilation of cisternae of the endoplasmic reticulum, swelling of mitochondria and detachment of the perinuclear membrane.
![]()
Fig 3 a
Tumor specimen obtained at 1 h after PDT with ZnOPPc (x 6,000). The neoplastic cells show a marked swelling of some mitochondria and different degrees of chromatin condensation.
Fig 3 b
Tumor specimen obtained at 3 h after PDT with ZnOPPc (x 9,000). The micrographs shows extensive vesciculations in the cell nucleus.
![]()
The morphological characteristics peculiar of apoptosis were observed with a much higher frequency in ZnOPPc- and even more in ZnODPc-photosensitized tumours: a rough estimate, based on the analysis of at least eight sections per mouse from three different mice, would indicate that at 1-3 h after PDT with ZnOPPc apoptosis and random cell death yield about the same contribution to the overall tumour damage, while the extent of apoptosis was significantly larger with ZnODPc as the photosensitizer. Moreover, while ZnOPPc appeared to induce an important direct damage of malignant cells at a faster rate as compared with other constituents of the tumour tissue, ZnODPc-photosensitized tumours showed both heavily damaged malignant cells and a remarkably altered vascular system already at 1 h after PDT (Fig. 4).
![]()
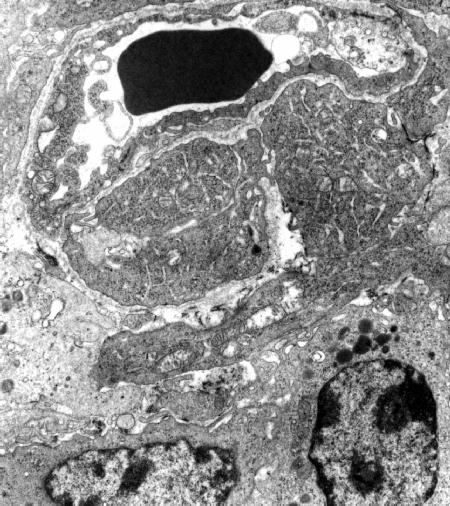
Fig 4
Tumor specimen obtained at 1 h after PDT with ZnDPPc (x 9,000). The endothelial and neoplastic cells appear to undergo an important damage at the level of both the endoplasmic reticulum and the mitochondria.
![]()
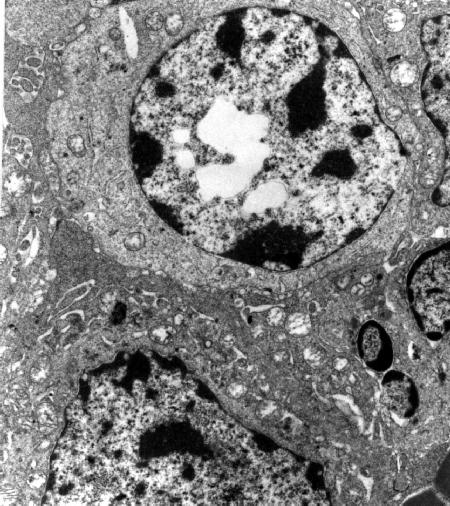
In all cases, the damage of the tumour tissue gradually progressed with time and at 24 h after PDT the organization of the neoplastic tissue was almost completely lost, and the cell borders were often vaguely defined owing to the extensive disruption of the cytoplasmic membrane (Fig. 5).
![]()
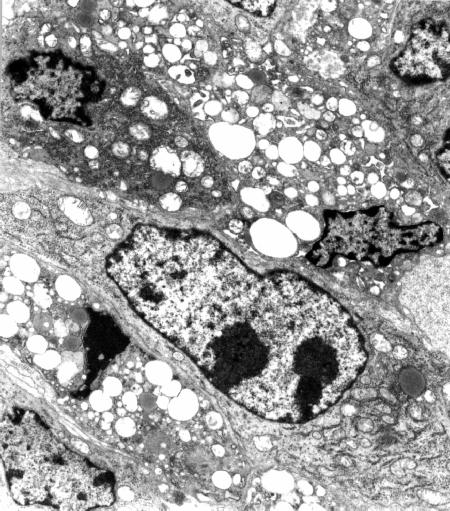
Fig 5
Tumor specimen obtained at 24 h after PDT with ZnPc (x 6,000). The neoplastic tissue appears completely disorganized, with several gaps in the cytoplasmic membrane and numerous vacuoles in the cytoplasm of neoplastic cells
![]()
4. Discussion
Our findings clearly indicate that the ZnPc and its octapentyl- and octadecyl-derivatives photosensitize the damage of the MS-2 fibrosarcoma by at least partially different mechanisms, as regards both the nature of the tissular districts which appear to be affected at short post-irradiation times and the mode of cell death (apoptosis vs. random necrosis) consequent to the PDT treatment. The three ZnPcs used in the present investigation show only minor differences in their pharamacokinetic behaviour, especially as regards the time-course and efficiency of accumulation in the tumour (see Table 1). Moreover, their photosensitizing activity should be comparable, since the quantum yield for generation of singlet oxygen is in the 0.5-0.7 range [10] , [20]. Thus, it is unlikely that photophysical or biodistribution properties are responsible for the observed differences in the response of the tumour upon irradiation in the presence of ZnPc and its derivatives.
A possible interpretation of our findings relies on a partially different subcellular and/or subtissular distribution of the three ZnPcs. Recent findings [21] , [22] actually suggest that the chemical structure can strongly influence the localization of phthalocyanines in tumour cells, and the onset of apoptotic responses is modulated by the type of interaction of a photosensitizer with adjacent targets. Our electron microscopy studies point out that membrane damage plays a major role in the ZnPcs-photoinduced alteration of cell morphology, a process which was shown to promote both delayed and arrested apoptosis; it is conceivable that the presence of relatively long alkyl chains favours the partitioning of the phthalocyanine in lipid domains, thereby initiating a cascade of events (e.g. activation of phospholipase C) resulting in the activation of endonucleases [23]. Additionally, a relocation of the ZnPcs even at very early times after the beginning of irradiation [22] could occur at different rates depending on their microenvironment, thus justifying the occurrence of different avenues for tumour necrosis.
The foregoing observations would indicate that the induction of random necrosis causes a more efficient tumour response to PDT than the induction of apoptosis. However, this conclusion obviously needs to receive further support from more detailed studies at a refined level concerning the role of specific cell organelles, especially membrane constituents, as determinants of apoptosis and random necrosis.
References
- T.J. Dougherty, Therapy and detection of malignant tumours, Photochem. Photobiol., 45 (1987) 879-889.
- S. Marcus, Clinical photodynamic therapy: the continuing evolution. In: B.W. Henderson, T.J. Dougherty (eds.), Photodynamic Therapy: Basic Principles and Clinical Applications, Marcel Dekker, New York, 1992, pp. 219-268.
- G. Jori, Factors controlling the selectivity and efficiency of tumour damage in photodynamic therapy, Laser Med. Sci., 5 (1990) 115-120.
- C. Zhou, Mechanisms of tumour necrosis induced by photodynamic therapy, J. Photochem. Photobiol., B:Biol., 3 (1989) 299-318.
- M.L. Agarwal, M.E. Clay, E.J. Harvey, H.H. Evans, A.R. Antunez, N.L. Oleinick, Photodynamic therapy induces rapid cell death by apoptosis in L5178Y mouse lymphoma cells, Cancer Res., 51 (1991) 5993-5996.
- X.Y. He, R.A. Spikes, S. Thomsen, L.W. Chung, L. Jacques, Photodynamic therapy with Photofrin II induced programmed cell death in carcinoma cell lines, Photochem. Photobiol., 59 (1994) 468-473.
- G. Jori, Far-red absorbing photosensitizers: their use in the photodynamic therapy of tumours, J. Photochem. Photobiol., B:Biol., 8 (1992) 371-378.
- J.E. van Lier, Phthalocyanines as sensitizers for photodynamic therapy of cancer. In: D. Kessel (ed), Photodynamic Therapy of Neoplastic Disease, Vol. I, CRC Press, Boca Raton, 1991, pp. 279-290.
- E. Ben-Hur, Basic photobiology and mechanisms of action of phthalocyanines. In: B.W. Henderson, T.J. Dougherty (eds.), Photodynamic Therapy: Basic Principles and Clinical Applications, Marcel Dekker, New York, 1992, pp. 63-78.
- M.J. Cook, I. Chambrier, S.J. Cracknell, D.A. Mayes, D.A. Russell, Octa-alkyl-zinc phthalocyanines: potential photosensitizers for use in the photodynamic therapy of cancer, Photochem. Photobiol., 62 (1995) 542-545.
- G. Valduga, E. Reddi, G. Jori, Spectroscopic studies on Zn(II)-phthalocyanine in homogeneous and microheterogeneous systems, J. Inorg. Biochem., 29 (1987) 59-65.
- M.J. Cook, C. Fabris, C. Ometto, D.A. Mayes, G. Jori, J. McMurdo, C. Milanesi, D.A. Russell, Highly substituted phthalocyanine derivatives as potential photosensitizers for photodynamic therapy of tumours. In: G. Jori, J. Moan, W.M. Star (eds.), Photodynamic Therapy of Cancer, Vol. 2078, Proc. SPIE, Bellingham, 1994, pp. 539-546.
- E. Reddi, G. Lo Castro, R. Biolo, G. Jori, Pharmacokinetic studies with Zn(II)-phthalocyanine in tumour-bearing mice, Br. J. Cancer, 56 (1987) 597-600.
- C. Milanesi, C. Zhou, R. Biolo, G. Jori, Zn(II)-phthalocyanine as a photodynamic agent for tumours. II. Studies on the mechanism of photosensitized tumour necrosis, Br. J. Cancer, 61 (1990) 846-850.
- C. Ometto, C. Fabris, C. Milanesi, G. Jori, M.J. Cook, D.A. Russell, Tumour-localising and -photosensitising properties of a novel zinc(II)-octadecylphthalocyanine, Br. J. cancer, 74 (1996) 1891-1899.
- C. Fabris, C. Ometto, C. Milanesi, G. Jori, M.J. Cook, D.A. Russell, Tumour-localizing and tumour-photosensitizing properties of zinc (II)-octapentyl-phthalocyanine, J. Photochem. Photobiol., B:Biol., 39 (1997) 279-284.
- G. Jori, Photodynamic therapy: a novel approach to the treatment of tumours, Bull. Mol. Biol. Med., 15 (1990) 73-83.
- G. Jori, E. Reddi, The role of lipoproteins in the delivery of tumour-targeting photosensitizers, Int. J. Biochem., 25 (1993) 1369-1375.
- B.W. Henderson, T.J. Dougherty, How does photodynamic therapy work?, Photochem. Photobiol., 55 (1992) 145-157.
- G. Valduga, S. Nonell, E. Reddi, G. Jori, S. Braslavsky, The production of singlet molecular oxygen by Zn(II)-phthalocyanine in ethanol and in unilamellar vesicles. Chemical quenching and phosphorescence studies, Photochem. Photobiol., 48 (1988) 1-5.
- D. Kessel, Y. Luo, Y. Deng, C.K. Chang, The role of subcellular localization in initiation of apoptosis by photodynamic therapy, Photochem. Photobiol., 65 (1997) 422-426.
- S.R. Wood, J.A. Holroyd, S.B. Brown, The subcellular localization of Zn(II)-phthalocyanines and their redistribution on exposure to light, Photochem. Photobiol., 65 (1997) 397-402.
- M.L. Agarwal, H.E. Larkin, S.I. Zaidi, H. Mukthar, N.L. Oleinick, Phospholipase activation triggers apoptosis in mouse lymphoma cells, Cancer Res., 53 (1993) 5897-5902.