EPR detection of reactive oxygen in the
photosynthetic apparatus of higher plants under light stress
╔va Hideg and Imre Vass
Institute of Plant Biology, Biological Research Center, Szeged, Hungary
INTRODUCTION
Plants performing oxygenic photosynthesis have developed a balanced system of enzymatic and non-enzymatic defence against reactive oxygen species (ROS), e.g. singlet oxygen, hydrogen peroxide or various oxygen free radicals. This way, although molecular oxygen and its highly reactive forms are continuously produced in illuminated chloroplasts, whose thylakoid membranes containing both highly unsaturated fatty acids – which can participate in free radical cascades –and excessive concentration of chlorophyll – a potential photosensitising dye –, the antioxidant system is usually sufficient to prevent damage under normal metabolic conditions. However, this balance is frequently disturbed in plants subject to unfavourable environmental conditions and/or pollutants. Activated oxygen has been implicated in the damage of plants upon numerous types of natural and artificial stress conditions (Asada et al 1994, Demmig-Adams and Adams 1994, Foyer et al 1994, Krause 1994, Hideg 1997).
Surprisingly, light — the obligatory driving force of higher plant photosynthesis — is among these stress factors. Under conditions when photochemically active radiation (PAR) is in excess, either due to unusually high intensity irradiation or as a consequence of lowered photon utilising capacity, reactive molecules capable of initiating membrane, protein and pigment damage are photoproduced. The complex set of these reactions is known as photoinhibition (PI, Powles 1984). If stress conditions are not severe, plants may prevent the above situation by facilitating energy utilisation, e.g. down regulating the energy input or by dissipating the excess energy (Allen 1992, Demmig-Adams and Adams 1992). Surplus PAR, however, may exhaust the adaptation systems (reviewed by Demmig-Adams 1992, Foyer and Harbison 1994, Krause 1994) and result in the overexcitation of photosynthesis. PI results in a net, in vivo decrease of photosynthetic activity. It is generally accepted, that the primary target of damage is photosystem (PS) II.
PS II is a pigment protein complex with a reaction centre consisting of a heterodimer of two membrane spanning proteins, D1 and D2. These either bind or contain the redox cofactors involved in the electron transport (Namba and Satoh 1987). The bound components are: the primary electron donor chlorophyll dimer (P680), the primary electron acceptor pheophytin (Pheo), the subsequent quinone electron acceptors QA and QB. Electrons from the catalytic cleavage of water by a manganese containing cluster bound to the lumenal side of D1/D2 are transferred to P680 via TyrZ, a redox active residue on D1. From P680, electrons are delivered to a mobile pool of plastoquinone molecules by subsequent redox reactions via Pheo, QA and QB (Scheme. 1).
![]()
Scheme 1
Photosynthetic electron transport in PS II of higher plants (see
explanation in text).
Oxygen evolving thylakoid membrane, PS II and other
sub-thylakoid membrane preparations provide good models for
studying light stress. These in vitro studies have revealed the
occurrence of two major routes, the so called acceptor side
induced and donor side induced photoinhibition (API and DPI,
respectively). Both API and DPI result in the impairment of PS II
electron transport followed by the selective degradation of the
D1 reaction centre protein (Mattoo et al 1984) and, to a lesser
extent, of the D2 protein (Schuster et al 1988). Prolonged PI
results in more general membrane damage, characterised by the
appearance of lipid peroxidation products (Hundal 1992, Hideg et
al 1994a) (Scheme 2). The two forms of PI are distinguished on
the basis of differences in the primary site of electron
transport malfunctioning, fragmentation pattern of the subsequent
D1 protein degradation, as well as in the light intensity and
oxygen requirement of the two process (for review see Aro et al
1993 and references therein). A third, alternative pathway of PI
has been suggested to operate under low light intensities. ROS
are also likely involved in this process, but their predicted
amount is below the dection level of methods available at present
(Keren et al 1995, 1997).
Both models of PI assume the formation of active oxygen (Aro et
al 1993, Telfer and Barber 1994 and references therein). In API,
which is caused by excess PAR in the presence of oxygen, singlet
oxygen production has been predicted as a result of increased
reaction centre chlorophyll triplet formation, which is a
consequence of the non-physiological over-reduction of the first
quinone electron acceptor in photosystem II (Vass and Styring
1992, Vass et al 1992, Aro et al 1993). DPI occurs when electron
flow from water to P680 is insufficient. There is a consensus
that the damage is triggered by the strong oxidants (P680+,
TyrZ+) created by primary charge separation and whose lifetime is
prolonged as a result of inoperative water splitting (Thompson
and Brudwig, 1988, Telfer and Barber 1989). In such case, both
electron transport and protein damage proceed in the absence of
oxygen even upon illumination with relatively lower intensities
of PAR (Jegersch÷ld and Styring, 1991).
Similarly to PI by excess PAR, UV-B (280-320 nm) irradiation
causes a multitude of physiological and biochemical changes in
plants, although these two types of light stress are different at
several points. Increased doses of UV-B radiation reaching the
Earth's surface as a consequence of stratospheric ozone depletion
have increased interest in this form of light stress in the past
decade. It is well established that UV-B results in the rapid
inactivation of photosynthetic electron transport, altered
pigment composition, destruction of the membrane structure and it
may cause the dimerisation of thymine bases and lesions in DNA
(for reviews see Tevini and Teramura 1989, Vass 1997). The
increased synthesis of flavinoids (Bornman 1989) – effective
quenchers of singlet oxygen, hydroxyl, superoxide and peroxy
radicals – as well as the increased expression of genes for
flavinoid biosynthesis (Strid 1993) imply the involvement of ROS
in the process. In the thylakoid membrane, the primary target of
UV-B is PS II: damage by UV-B involves functional impairment of
PS II electron transport (Kulandaivelu and Noorudeen 1983, Renger
et al 1989, Hideg et al 1993) and degradation of PS II reaction
centre proteins, primarily D1 (Renger et al 1989, Greenberg et al
1989, Friso et al 1994a) and D2 (Friso et al 1994b).

Scheme 2
Scematic representation of the main events of light
stress in plants.
Our in vitro studies have confirmed production of ROS in the above light stress conditions. The aim of the present work is to review these studies and to show possible outlooks on in vivo applications. Figures 4, 5 and 6 contain unpublished data, which will be published in the printed, journal version of the First Internet Conference on Photochemistry and Photobiology.
PRODUCTION OF ROS IN VITRO
The triplet chlorophyll – singlet oxygen model of API was evidenced by observation of reaction centre chlorophyll triplet quenching by oxygen (Vass and Styring 1992, Vass et al 1992) and direct detection of singlet oxygen (Hideg et al 1994b). Our studies have shown that singlet oxygen production is a unique characteristic of API among the three studied forms of light stress (Hideg et al 1994a, 1994b, Hideg and Vass 1996) (Fig. 1) and it originates in PS II (Hideg and Vass 1995) (Fig. 2).
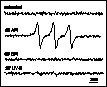
Figure 1
EPR detection of singlet oxygen trapped by TEMP in
thylakoid membranes: untreated, exposed to 15 min PI (API),
or to 35 min UV-B irradiation and in Tris pre-treated
thylakoid membranes photoinhibited for 15 min (DPI). TEMPO
is detected as aN = 1.52 mT.
Singlet oxygen may trigger the subsequent D1 protein damage, either directly or by promoting a special confirmational change which makes the protein susceptible for proteolytical damage. The possibility of a direct cleavage by singlet oxygen or by the products of a singlet oxygen induced radical cascade has been supported by the observation of D1 protein degradation without the conditions of photoinhibition, indused by the chemical or photodynamic generation of singlet oxygen (Mishra and Ghanotakis 1994).

Figure 2
EPR detection of singlet oxygen trapped by TEMP in PS
II and PS I thylakoid membrane sub-preparations upon exposure to
PI for 25 min.
Besides the role of this non-radical form of active oxygen in API, the involvement of oxygen free radicals is feasible on the basis of models on the events of both API and DPI pathways (for review see Aro et al 1993 and references therein). The possibility of radical formation has been supported by the lessening of photoinhibition induced damage in samples containing various free radical scavengers and/or antioxidant enzymes (Sopory et al 1990, Richter et al 1990). PI induced free radical production has been evidenced by spectrophotometry in-non-oxygen-evolving PS II preparations (Chen at al. 1992, 1995) and also directly, by EPR spectroscopy in thylakoid membranes (Hideg et al 1994a) and in Chlorella (Hirayama et al 1995). In DPI, mainly hydroxyl radicals (Fig. 3, Hideg et al 1994a) and superoxide radicals (Fig.5, Chen et al 1992) are produced. API is dominated by carbon-centred radicals (Fig. 3, Hideg et al 1994a), but hydroxyl radicals may also contribute in the latter phase of damage (data not shown, Hideg et al 1994b, Shiraishi et al 1994). In line with reports on photoinhibitory D1 protein damage in the absence of oxygen during DPI (Jegersch÷ld and Styring 1991) but not in API (Hundal et al 1990), we found that free radical production required the presence of oxygen during API but not in DPI (Fig. 3), demonstrating that ROS production and the propagation of PI are closely related.
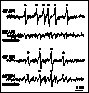
Figure 3
EPR detection of free radicals by DMPO in thylakoid membranes or
Tris pre-treated thylakoid membranes upon exposure to PI for 30
min (realisation of API and DPI, respectively). Experiments were
carried out both in the presence (no label) and
absence(„anaerobic") of oxygen. EPR spectra were best
interpreted with the following hyperfine splitting constants: aN = abetaH = 1.47 mT
for DMPO-OH ( * ) and aN = 1.65 mT,
abetaH = 2.32 mT
for DMPO-CH3 ( o ).
UV-B irradiation also results in free radical (Fig. 4) but not in singlet oxygen production (Fig 1). The latter observation demonstrates that the primary site of UV-B induced electron transport impairment is different from that of PI by excess PAR. In thylakoid membranes, UV-B irradiation results in parallel production of several, mainly hydroxyl and carbon centred free radicals (Hideg and Vass, 1996). Comparing a series of samples with increasing PS II purity — thylakoid membranes, PS II and PS II core complex preparations — suggests that the primary event is hydroxyl radical production in PS II, the other observed free radicals are probably produced in reactions initiated by these primary products.

Figure 4
EPR detection of free radicals by DMPO in thylakoid
membranes and two sub-thylakoid preparations upon UV-B
irradiation for 30 min. Hyperfine splitting constants: aN = abetaH = 1.47 mT
for DMPO-OH ( * ) and aN = 1.65 mT,
abetaH = 2.32 mT
for DMPO-CH3 ( o ).
Superoxide anion radicals are formed as by-products during the operation of photosynthetic electron transport (Mehler, 1951). Their production site is at PS I, where molecular oxygen provides an alternative sink for electrons in illuminated thylakoid membranes in the absence of NADP (Takahashi and Asada 1988, Asada 1992). Accordingly, superoxide radicals can be trapped in isolated thylakoid membranes with Tiron (Fig. 5, Hideg et al 1995). This signal is inhibited by DCMU — which blocks the electron transport between PS II and PS I —, increased by the addition of methyl-viologen — enhancer of the Mehler reaction (Takahashi and Katoh 1984, Shiraishi et al 1992) — (data not shown, Hideg et al 1995), and no superoxide trapping was observed from the PS II core complex, even under conditions of API (Fig. 5). These results demonstrate that the source of the observed EPR signal is indeed the trapping of superoxide radicals from PS I by Tiron. Also, API only slightly enhanced the EPR signal in thylakoid membranes, indicating that superoxide radicals are not the main promoters of API (Fig. 5, Hideg et al 1995). On the other hand, DPI resulted in intense superoxide production (Fig. 5) in PS II core complexes, in accordance with previous suggestions (Chen et al 1992, 1995). Superoxide radicals are also produced in thylakoid membranes and PS II preparations exposed to UV-B irradiation (data not shown), but sensitivity of Tiron itself to UV-B irradiation does not allow precise conclusions.

Figure 5
EPR detection of superoxide radicals trapped with
Tiron in thylakoid membranes (untreated and 30 min exposure
to API) and in PS II core complex preparations exposed to
either API or DPI for 30 min.
Conclusions
Table 1 summarises results of our in vitro ROS detection studies. As it is shown here, singlet oxygen production appears as a unique characteristic of API, providing a possibility for in vivo identification of the process (see below). Although hydroxyl radical production is a common characteristic of DPI and stress by UV-B irradiation, further details of the two light stress suggest distinct mechanism.
EPR detection of singlet oxygen is in line with the model which has been suggested earlier (Vass et al 1992, Vass and Styring 1992), that in API, the singlet oxygen molecules produced in impaired photosynthetic reaction centres participate in the initiation of the subsequent D1 protein damage. However, there is still no consensus on the role of free radicals in light stress. It is not yet clear whether the free radicals observed are degradation by-products, initiators or promoters of the protein and membrane lipid damage.
ROS IN VIVO
The above results indicate that ROS probably also play an important role in light stress in vivo. However, direct extension of in vitro spin trapping techniques to in vivo studies meets experimental difficulties. As it is shown in Fig. 6, infiltration of the trap 2,2,5,5-tetramethyl-pyrrolidine into intact leaves indicates the occurrence of singlet oxygen production. However, the nitroxide radical PROXYL is rapidly metabolised in the leaf and its conversion hampers in vivo singlet oxygen detection studies, especially quantitative ones. Extraction of the hydroxylamine product of such reactions into an organic solvent and its conversion back to the EPR active PROXYL may solve this problem (data not shown) but makes quantitative in vivo studies difficult.
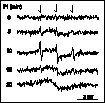
Figure 6
EPR absorption spectra of
2,2,5,5-tetramethyl-pyrrolidine infiltrated broad bean leaf
segments after exposure to API by 2000 micromol m-2 s-1
PAR for times indicated in the figure. PROXYL radicals (downward
arrows) were observed with aN = 1.51 mT
hyperfine spliting.
Free radical detection is even more obstructed in vivo. Ascorbate, which is present at high concentrations in leaves as well as other reducing agents react with DMPO spin adducts yielding EPR silent compounds. Rapid post-stress isolation of thylakoid membranes in the presence of the spin trap helps this situation, as it is illustrated by the example of detecting free radicals in UV-B exposed leaves (Fig. 7). Nevertheless, it is important to note, that while this post-stress detection technique provides evidence for the occurrence of oxidative stress, the trapped free radicals are very likely not the primary in vivo products of such damage. On the other hand, increased quantities of ascorbate (monodehydroascorbate) radicals in the supernatant fraction after UV-B stress (Fig. 7) reveal the possibility of utilising these radicals as natural, built-in stress markers (Hideg et al 1997). In vivo, in situ detection of ROS is necessary in order to understand the mechanism of light stress – as well as that of other plant stress situations. Application of the spin trapping EPR spectroscopy technique, which has been proved useful in in vitro experiments, offers an insight into such studies already. Improvement of spin traps and ROS sensors will provide an opportunity to judge whether pathways identified in in vitro are relevant to field conditions and might help finding more stress tolerant biotypes among agricultural plants.
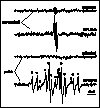
Figure 7
EPR spectra of pellet (thylakoid) and supernatant
fractions of crude leaf extracts from untreated and UV-B treated
broad bean leaves prepared in the presence of DMPO. Two line EPR
spectra in the supernatant fraction are from
monodehydro-ascorbate radicals. The spectrum observed in the
pellet fraction was best interpreted as a sum of spectra from
various DMPO radical adducts with the following hyperfine
splitting constants: aN = abetaH = 1.47 mT
for DMPO-OH ( * ), aN = 1.65 mT,
abetaH = 2.32 mT
for DMPO-CH3 ( o ) and aN = 1.65 mT,
abetaH = 1.07 mT,
agammaH = 0.14 mT
for the peroxy radical adduct DMPO-OR ( # , two
lines of the 6-line spectrum are hidden).
ACKNOWLEDGEMENTS
Our experimental studies concerning the topic of this review have been supported by research grants from the Hungarian Scientific Research Fund, OTKA F-6241 (1993-1995) and T-17455 (1994-1998).
EXPERIMENTAL PROCEDURES
Samples
Thylakoid membranes were prepared from market spinach
according to the method of Takahashi and Asada (1982),
re-suspended in a buffer containing 40 mM Hepes
(pH 7.2), 0.4 M sucrose, 15 mM NaCl and 5 mM
MgCl2, and kept on ice in the dark until use.
Tris-washing, which removes Mn and the water soluble subunits of
the water splitting enzyme was performed according to Wydrzynski
et al (1985). Tris-treated thylakoids were pelleted by
centrifugation, re-suspended under dim light in the above Hepes
buffer containing 20 mM NaHCO3 and stored on ice
in the dark until use. As a result of this treatment, the oxygen
evolving ability of the samples was decreased by more than 85%.
PS II enriched (BBY-type) preparations were made according to a
slightly modified version of the method described by Berthold et
al (1981), stored and measured in 50 mM Mes (pH 6.0),
0.4 M sucrose, 10 mM NaCl and 5 mM MgCl2.
PS II core complexes were prepared according to Ghanotakis et al
(1987) and kept in a buffer containing 20 mM Mes (pH 6.0),
0.4 M sucrose, 10 mM NaCl, 5 mM CaCl2
and 2 mM dodecyl-maltoside until use.
PS I enriched membrane preparations (PSI-110-type particles)
were made from thylakoid membranes according to the method of
Mullet et al (1980), resuspended and measured in a buffer
containing 0.1 M sucrose, 25 mM Hepes (pH 7.8),
10 mM NaCl and 5 mM MgCl2.
Anaerobic conditions were achieved by bubbling the samples with
argon. Chlorophyll content was determined according to Arnon
(1949).
Light stress
In order to achieve the conditions of API, thylakoid membranes, PS II or PS II core complex preparations were diluted to 100 microgram chlorophyll / mL with the corresponding buffer, without any external electron acceptor and illuminated by 1000-1500 micromol m-2 s-1 PAR from a KL-1500 (DMP, Switzerland) lamp through an optical fiber guide in a 1 cm cuvette from the side. DPI was studied either in PS II core complex preparations at pH 8.0 (Tris-HCl buffer instead of Mes) in the presence of 0.2 mM DBMIB as electron acceptor or in Tris-washed thylakoids at pH 7.2 (Hepes thylakoid buffer) under the same light conditions as above. UV-B irradiation was provided by a VL-215M lamp (Vilbert Lourmat, France) with maximal emission at 312 nm and 20 micromol m-2 s-1 total UV-B intensity, as measured with a Cole-Parmer 97503-00 radiometer (Cole Parmer, USA). Samples were irradiated from above through a cellulose acetate film (Courtaulds Chemicals, UK) to remove UV-C radiation. 200 microL volumes of isolated photosynthetic membranes were irradiated in corresponding buffer solutions in a 1 cm cuvette from above. In in vivo experiments 6 weeks broad bean (Vicia faba L.) leaves were used. For both PI and UV-B irradiation experiments, detached leaves were placed on wet tissue paper with their adaxial sides up, under moderate air flow from a fan. This method assured that leaves were neither dried nor heated during the treatment. When indicated, leaves were infiltrated with a 60 mM aqueous solution of 2,2,5,5-tetramethyl-pyrrolidine. Leaf segments (2 x 10 mm) were cut from the middle of part of the leaf (avoiding the midrib) immediately after cessation of strong illumination and put in the EPR spectroscope in a quartz tube.
ROS detection
Free radicals – contrary to the majority of biomolecules – are paramagnetic. This offers EPR spectroscopy an ideal tool for their detection. However, since free radicals are short-lived and present at very low concentrations, spin traps are applied to facilitate their detection. Spin trapping EPR spectroscopy is based on the reaction between a diamagnetic compound (the spin trap) and the free radical yielding a paramagnetic and relatively stable product (Scheme 3). Free radicals were trapped with 67 mM DMPO. DMPO is the most useful trap for the study of oxygen-centred free radicals due to its many adducts with distinct EPR spectra (Janzen and Liu 1973). However, DMPO trapping may underestimate the amount of superoxide radicals in the sample, due to the conversion of DMPO-OOH into DMPO-OH (Finkelstein et al 1979, 1980). The use of Tiron, a quinone compound yielding a stable quinone radical upon reacting with superoxide may provide a better solution for studying superoxide production (Greenstock and Miller 1975, Miller and MacDowall 1975).
![]()
Scheme 3
Principle of spin trapping (see details in text).
Similarly to free radical trapping, singlet oxygen, the
non-radical form of reactive oxygen was detected utilising TEMP, which forms the nitroxide radical TEMPO with singlet oxygen (Lion et al 1976,
1980). Samples contained 10 mM TEMP. Singlet oxygen
detection with TEMP may be jeopardised by the conversion of the
produced TEMPO into EPR silent hydroxylamine by the reducing
compounds formed in the thylakoid membrane. In order to avoid
this situation, samples were extracted after the light stress
treatment into ethylacetate and aired in the presence of
catalytic amounts of PbO2 before EPR spectroscopy, as
described earlier (Hideg and Vass, 1993). Conversion of
2,2,5,5-tetramethyl-pyrrolidine into the nitroxide PROXYL upon reaction with singlet oxygen
was utilised in leaves similarly to that of TEMP in in vitro
studies.
EPR spectra were measured with a Bruker ECS-106 spectrometer.
X-band spectra were recorded at room temperature with
9.45 GHz microwave frequency, 16 mW power, 100 kHz
modulation frequency and 0.2 mT modulation amplitude, as
described earlier (Hideg and Vass, 1993, Hideg et al 1994a,
1994b). In order to insure comparative EPR quantitation, all
spectra of spin traps in samples were measured under identical
experimental conditions, at uniform 15 microL volumes in
uniform glass capillaries. EPR gain parameters were kept uniform
in experiments illustrated on the same figure.
REFERENCES
Allen, J.F. (1992) Biochim. Biophys. Acta 1098, 275-335
Arnon, D.I. (1949) Plant Physiol. 24, 1-25.
Aro, E.-M., Virgin, I. and Andersson, B. (1993) Biochim. Biophys.
Acta 1143, 113-134
Asada, K. (1992) In: Molecular Biology of Free Radical Scavenging
Systems (Ed. Scandalios, J.G.), pp. 173-192, Cold Spring Harbor
Laboratory Press, New York
Asada, K. (1994) In: Causes of Photooxidative Stress in Plants
and Amelioration of Defence Systems (Eds.: Foyer, C. H. and
Mullineaux, P.M.) pp. 77-104, CRC Press, Florida, USA
Berthold, D.A., Babcock, G.T. and Yocum C.F. (1981) FEBS Lett.
134, 231-234.
Bornman, J.F. (1989) J. Photochem. Photobiol. B. Biol. 4, 145-158
Chen, G.-X., Kazimir, J. and Cheniae, G.M. (1992) Biochemistry
31, 11072-11083
Chen, G.-X., Blubaugh, D.J., Homann, P.H., Golbeck, J.H. and
Cheniae, G.M. (1995) Biochemistry 34, 2317-2332
Demmig-Adams, B. and Adams III, W.W. (1992) Annu. Rev. Plant
Physiol. Plant Mol. Biol. 43, 599-626
Demmig-Adams, B. and Adams III, W.W. (1994) In: Causes of
Photooxidative Stress in Plants and Amelioration of Defence
Systems (Eds.: Foyer, C. H. and Mullineaux, P.M.) pp. 105-126,
CRC Press, Florida, USA
Finkelstein, E., Rosen, G.M. and Rauckman, E.J. (1979) Mol.
Pharmacol. 16, 676-685
Finkelstein, E., Rosen, G.M. and Rauckman, E.J. (1980) J. Amer.
Chem. Soc. 102, 4994-4999
Foyer, Ch.H. and Harbison, J. (1994) In: Causes of Photooxidative
Stress and Amelioration of Defence Systems in Plants (Eds.,
Foyer, Ch.HH. and Muullineaux, Ph.M.) pp.1-42, CRC Press, Boca
Raton, Ann Arbor, London, Tokyo
Foyer, Ch.H., Lelandais, M. and Kunert, K.J. (1994) Physiol.
Plant. 92, 696-717
Friso, J., Spetea, C., Giacometti, G.M., Vass, I. and Barbato, R.
(1994a) Biochim. Biophys. Acta, 1184, 78-84
Friso, J., Barbato, R., Giacometti, G.M. and Barber, J. (1994b)
FEBS Lett. 339, 217-221
Ghanotakis, D.F., Demetriou, D.M. and Yokum, C.F. (1987) Biochim.
Biophys. Acta 891, 15-21
Greenberg, B.M., Gaba, V., Canaani, O., Malkin, S. and Edelman,
M. (1989) Proc. Natl. Acad. Sci. USA, 86, 6617-6620
Greenstock, C.L. and Miller, R.W. (1975) Biochim. Biophys. Acta
396, 11-16
Hideg, ╔. and Vass, I. (1993) Photochem. Photobiol. 58, 280-283
Hideg, ╔., Sass, L., Barbato, R. and Vass, I. (1993) Photosynth.
Res. 38, 455-462
Hideg,╔., Spetea,C. and Vass,I. (1994a) Biochim. Biophys. Acta
1186, 143-152
Hideg,╔., Spetea,C. and Vass,I. (1994b) Photosynth. Res. 39,
191-199
Hideg, ╔. and Vass, I. (1995) Photochem. Photobiol. 62, 949-952
Hideg, ╔. and Vass, I. (1996) Plant. Sci. 115, 251-260
Hideg, ╔., Mano, J., Ohno, Ch. and Asada, K. (1997) Plant Cell
Physiol. 38, 684-690
Hideg, ╔. (1997) In: Handbook of Photosynthesis (ed. Pessarakli,
M.) pp. 911-930, Marcel Dekker Inc., New York
Hirayama, Sh., Ueda, R. and Sugata K. (1995) Free Rad. Res.
Commun. 23, 51-59
Hundal, T., Aro, E.-M., Carlberg, I. and Andersson, B. (1990)
FEBS Lett. 267, 203-206
Hundal,T. (1992) Ph.D. Thesis, Stockholm University,
ISBN-91-7153-068-1, Akademitryck AB, Edsbruk
Janzen, E.G. and I-Ping Liu, J. (1973) J. Magn. Reson. 9, 510-512
Jegersch÷ld, C. and Styring, S. (1991) FEBS Lett. 280, 87-90.
Keren, N., Gong, H. and Ohad, I. (1995) J. Biol. Chem. 270,
806-814.
Keren,N., Berg, A., van Kan, P.J.M., Levanon, H. and Ohad, I.
(1997) Proc. Natl. Acad. Sci. USA 94, 1579-1584.
Krause, G.H. (1994) In: Causes of Photooxidative Stress and
Amelioration of Defence Systems in Plants (Eds., Foyer, Ch.H. and
Mullineaux, Ph.M.) pp.43-76, CRC Press, Boca Raton, Ann Arbor,
London, Tokyo
Kulandaivelu, G. and Noorudeen, A.M. (1983) Physiol. Plant 58,
389-394
Lion, Y., Delmelle, M. and van de Vorst, A. (1976) Nature 263,
442-443
Lion, Y., Gandin, E. and van de Vorst, A. (1980) Photochem.
Photobiol. 31, 305-309
Mattoo, A.K., Hoffman-Falk, H., Marder, J.B. and Edelman, M.
(1984) Proc. Natl. Acad. Sci. USA 81, 1380-1384
Mehler, A.H. (1951) Arch. Biochem. Biophys. 33, 65-77
Miller, R.W. and MacDowall, F.D.H. (1975) Biochim. Biophys. Acta
387, 176-187
Mishra, N.P., Mishra, R.K. and Singhal, G.S. (1993) J. Photochem.
Photobiol. B: Biol. 19, 19-24
Mullet, J.E., Burke, J.J. and Arnzten, C.J. (1980) Plant Physiol.
65, 814-822
Namba, O. and Satoh, K. (1987) Proc. Natl. Acad. Sci. USA 84,
109-112
Powles,S.B. (1984) Annu. Rev. Plant Physiol. 35, 15-44.
Renger,G., Volker,.M., Eckert, H.J., Fromme, R., Hohm-Veit, S.
and Graber, P. (1989) Photochem. Photobiol. 49, 97-105.
Richter, M., RŘhle, W. and Wild, A. (1990) Photosynth. Res. 24,
237-243
Schuster, G. Timberg, R. and Ohad, I. (1988) Eur. J. Biochem.
177, 403-410.
Shiraishi, T., Takahashi, M.-A. and Asada, K. (1992) In: Research
in Photosynthesis (Ed. Murata, N.) Vol.II, pp.627-634, Kluwer
Academic Publishers, The Netherlands
Shiraishi, T., Takahashi, M.-A. and Asada, K. (1994) In:
Frontiers of Reactive Oxygen Species in Biology and Medicine
(Eds.: Asada, K. and Yoshikawa, T.), pp.31-32, Kluwer Academic
Publishers
Sopory, S.K., Greenberg, B.M., Mehta, R.A., Edelman, M. and
Mattoo, A.K. (1990) Z. Naturforsch. 45C, 412-417
Strid, A. (1993) Plant Cell Physiol. 34, 949-953
Takahashi, M. and Asada, K. (1982) Plant Cell Physiol., 23,
1457-1461
Takahashi, Y. and Katoh, S. (1984) Plant Cell Physiol. 25,
785-794.
Takahashi, M.A. and Asada, K. (1988) Arch. Biochem. Biophys. 26,
714-722
Telfer, A. and Barber, J. (1989) FEBS Lett. 246, 223-228
Telfer, A. and Barber, J. (1994) In: Photoinhibition of
Photosynthesis (Eds.: Baker, N.R. and Bowyer, J.R.) Ch.2,
pp.25-49, Bios Scientific Publishers, Oxford
Tevini, M. and Teramura, A.H. (1989) Photochem. Photobiol. 50,
479-487
Thompson, L.K. and Brudvig, G.W. (1988) Biochemistry 27,
6653-6658
Vass, I. and Styring, S. (1992) Biochemistry 31, 5957-5963
Vass, I., Styring, S., Hundall, T., Koivuniemi, A., Aro, E.-M.
and Andersson, B. (1992) Proc. Natl. Acad. Sci. USA 89, 1408-1412
Vass, I. (1997) In: Handbook of Photosynthesis (ed. Pessarakli,
M.) pp. 931-949, Marcel Dekker Inc., New York
Wydrzinski, T., Huggins, B.J. and Jursinic, P.A. (1985) Biochim.
Biophys. Acta 809, 125-136.
