PHOTOREACTIVITY AND SYSTEMIC SIDE-EFFECTS OF DRUGS
Nitroaromatics (Photoreactivity of Nifedipine in vitro and in vivo )
Henk de Vries and Gerard M.J. Beijersbergen van Henegouwen
Department of Medicinal Photochemistry
Leiden/Amsterdam Center for Drug Research, University of Leiden
P.O. Box 9502, 2300 RA Leiden, The Netherlands
Key-words: Nifedipine, UVA, photodegradation, photoallergy, immunotoxicity, nitroso derivative.
Abbreviations: BSA, Bovine serum albumin; GSH, glutathione; NHNIF, lactam derivative of NIF; NIF, nifedipine; NONIF, nitroso derivative of NIF; PEG, polyethylene glycol
Abstract
In this article reference is made to an extensive review on phototoxicity of drugs. The nitroaromatics are taken as an example and some emphasis is put on possible systemic phototoxic effects.
More in detail, in vivo and in vitro experiments are described regarding nifedipine, a nitroaromatic as well. Both in vitro and in vivo with rats exposed to UVA, a lactam derivative of nifedipine is formed. From in vitro data, it is tentatively concluded that irreversible binding to biomacromolecules occurs as well.
1. Introduction
Recently, an extensive review has been published on phototoxicity of drugs and other chemicals [1]. The reader is referred to this for a general introduction and for a detailed description of specific examples.
The review [1] has been written from a medicinal chemical point of view, which aims at insight into the relation between the molecular structure of a drug and its phototoxic effect. The fact is that with this kind of knowledge, we have the opportunity to alter the molecular structure of a drug in such a way that its phototoxicity diminishes whereas its desired pharmacological properties remain conserved. A prerequisite to reach this eventual aim is a combination of in vitro and in vivo research of the molecular processes which a given phototoxicon undergoes after absorption of UV or visible light. An example of this is the research with imino-N-oxides [1]. Chlordiazepoxide (e.g Librium) showed to be phototoxic because of the N-O group in the molecule; its analogue without the oxygen had the same pharmacological properties, but proved to be not phototoxic. The other way round was shown with diazepam (e.g Valium); if this non-phototoxic drug was oxidized to an imino-N-oxide, a phototoxic product was obtained.
A second point which got particular attention in this review [1] is the concept that photoreactivity of a drug can affect other organs than the skin and eyes which are exposed to light: systemic phototoxic effects. The possibility of the latter has also been investigated with nitroaromatics to which belongs nifedipine, of which we describe our latest results in the present article.
Nitroaromatics are key-compounds in chemical industry [2]; they are used to make several thousand consumer products, amongst which are important drugs. Toxic effects connected with their use are assumed to be caused by incomplete enzymatic reduction of the nitro group. This results in reactive intermediates such as nitroso and hydroxylamino compounds as well as free radicals [3], which can irreversibly bind to proteins, lipids and nucleic acids. (Irreversible binding of reactive intermediates to such biomolecules, is considered as one of the general mechanisms of toxic action [4, 5]. Besides, not only reactive intermediates but also stable end-products or metabolites of a drug or chemical can be toxic; for example by interference with normal receptor-ligand interactions [4].)
In general, photoactivation of nitroaromatics is not considered as a cause of their toxic effects [6]. This is remarkable if one considers their UVA and visible light absorbing capacity and the extent to which this radiation can penetrate the body.
The latter means that even systemic toxic effects resulting from photoactivation of nitroaromatics should be considered as a possibility. This is supported by research of the in vivo photoreactivity of nitroaromatics [1].
With regard to photoreactivity of nitroaromatics, it was shown that although reduction of the nitro group occurs, e.g. with musk ambrette and nifedipine, it is not a common rule. Other mechanisms such as nucleophilic substitution [7] and bond fission, e.g. chloramphenicol and nitrofurantoin, also occur [1]. In these cases, the nitro group does not react itself but has a distinct influence on the photoreaction by its electron withdrawing capacity.
Especially the results found with chloramphenicol and nitrofurantoin (NFT) are remarkable because they indicate the occurrence of systemic effects. With NFT, far more irreversible binding was found with UVA-exposed animals than with those kept in subdued light [1]. The irreversibly bound fragments of nitrofurantoin were not only found in the light exposed skin but also in the blood, spleen and lungs. This observation is important because side-effects of NFT are frequently of the allergic type and concern also the lungs. Photoadducts between NFT and plasma proteins have immunogenic properties: produced in vitro and injected into the same animals of which plasma was taken, they showed to induce the formation of antibodies. Besides, leucocytes taken from rats and exposed to the combination of NFT and UVA, were able to produce systemic immunosuppression in this animal model for photopheresis [1].
Nifedipine (NIF), an important drug used for the treatment of myocardial ischemia and hypertension, is extremely sensitive to ultraviolet radiation and to visible light upto 450 nm. The quantum yield for photodegradation is ~0.5; statistically this means that of every two photons absorbed, one causes decomposition of a NIF molecule. This extreme photoinstability added to the fact that NIF is frequently prescribed for a prolonged therapy, was the reason to start research as described above for other nitroaromatics [1].
In a previous study [8], it was found that UVA and daylight exposure give the same photoproducts. In phosphate buffer, the conversion is quantitative and the nitroso derivative of NIF (NONIF) was the only photoproduct (more than 99%).
However when NIF is irradiated in the presence of glutathione (GSH), in plasma and in blood, NONIF is a short-lived intermediate only. With 10-3 M GSH as the only reactant present in phosphate buffer pH 7.4, a lactam (NHNIF) is formed quantitatively (Fig. 1). The fact that NONIF is an instabile intermediate in the photoreaction with GSH was proven as follows. NIF was first converted into NONIF by UVA, without GSH being present. Subsequently, GSH was added and within 10 seconds of incubation in the dark, the same percentage of NHNIF (more than 99%) was formed as obtained during simultaneous irradiation of NIF with GSH.
As NIF is complexed with plasma proteins for ~95%, its photolability and the high reactivity of its first and main photoproduct NONIF, implies the possibility of irreversible photobinding to proteins.
In the present study, we investigate the possible formation of NHNIF in the rat exposed to UVA and address the question whether the photoreactivity of NIF also results in irreversible binding.
2. Materials and methods
2.1. Solvents and chemicals
All organic solvents used were of analytical grade and distilled before use. Water was demineralized and further purified in a "Milli-Q UF plus" water purification system (Millipore, Etten-Leur, The Netherlands). Nifedipine and nitrendipine were a gift from Bayer Nederland bv, Mijdrecht, The Netherlands, and used as received. Glutathione, disodium hydrogen phosphate, potassium dihydrogen phosphate, sodium sulphate exic., sodium chloride, hydrochloric- and phosphoric acid, polyethylene glycol (PEG) and glycerin were purchased from Merck, Darmstadt, F.R.G. PEG was mixed with 5.3% glycerin and 8.9% water; for convenience this mixture will be referred to as "PEG+". Bovine serum albumin (BSA) and mixed alkyltrimethylammoniumbromide (Cetrimide) were purchased from Sigma, St Louis, U.S.A. The nitroso- and lactam derivative of NIF were synthesized as previously described [8].
2.2. Preparation of animals
Male Wistar derived rats (200-220 g ; Charles River, Sulzfeld, Germany) were anaesthetized by a subcutane hypnorm (Janssen, Beerse, Belgium) injection and an intraperitoneal nembutal (Sanofi, Maassluis, The Netherlands) injection and placed on a thermostated plate (38o C). Subsequently, their back was shaven.
All in vivo experiments were approved by the Animal Experiments Commission of the Leiden University.
2.3. HPLC analysis
Samples were analysed using a 2150 HPLC pump (LKB, Bromma, Sweden) equipped with a Microspher C18, 100 x 4.6 mm column packed with 3 痠 particles (Chrompack, Middelburg, The Netherlands). Samples were injected with a Promis auto sampler (Separations, H.I.Ambacht, The Netherlands) supplied with a 20 無 injection loop. 25 mM Cetrimide in distilled water (pH 4.9)-acetonitrile (62:38) was used as an eluent (0.5 mL/min) for quantitative purposes. For qualitative analysis, gradient elution (0.5 mL/min) was applied with the acetonitrile percentage in the eluent changing from 10 to 90 % in 20 minutes. Detection took place with a Spectra Focus detector (Thermo separation products, Breda, The Netherlands) set at 240 nm for quantitative determination. Besides, spectra were recorded from 210 to 360 nm with the same apparatus for qualitative analysis.
Samples were put on pH 1 with 1N hydrochloric acid and extracted with chloroform. For quantitative analysis nitrendipine was added as an internal standard. The chloroform extracts were dried on sodium sulphate exic., evaporated under vacuo and dissolved in the HPLC eluent after which analysis took place.
All samples were kept in 2 mL brown glass vials (PhaseSep, Waddinxveen, The Netherlands) and processed in low actinic red light.
2.4. Irradiation conditions
For UVA exposure of the rats, an array of five TL 80 W/10 R lamps (Philips, Eindhoven, The Netherlands) was used. Spectral output of the lamps was 345 to 410 nm, with a maximum at 370 nm. The light intensity was 125 W/m2 measured on the level of the rats with a UVX radiometer (Ultraviolet Products Ltd., San Gabriel, USA) equipped with a UVX-36 sensor.
For UVA exposure of the samples, an array of six TL 80 W/10 R lamps (Philips, Eindhoven, The Netherlands) was used. Spectral output of the lamps was 345 to 410 nm, with a maximum at 370 nm. Samples were put in the "merry-go-round" of which the axis was at 40 cm distance from the array of lamps. The latter was placed in a vertical position with the tubes perpendicular to the axis of the "merry-go-round". Average intensity of the light was 50 W/m2, as measured at the axis of the "merry-go-round" with the UVX radiometer equipped with a UVX-36 sensor. Samples were exposed in clear glass vials (UV cutoff at 275 nm) of 11.5 mm diameter (PhaseSep, Waddinxveen, The Netherlands).
2.5. Clearance of NHNIF from the blood
The iliac vena and artery of four prepared rats were cannulated. They were given an intravenous injection of 250 痢 of NHNIF in 25 無 of PEG+. One minute after application of the dose, a blood-sample of 300 無 was taken and this was repeated after 5, 10, 15, 20 and 30 minutes. The samples were diluted with 500 無 water, centrifuged at 2000 g and the supernatants analyzed as described in 2.3.
2.6. Biliary excretion of NHNIF
The iliac vena and bile-duct of four prepared rats were cannulated. At t=0 min, they were given an intravenous injection of 250 痢 of NHNIF in 25 無 of PEG+. Thereafter, the bile produced during the period 0-10, 10-20, 20-30, 30-40, 40-50 and 50-60 min was collected. After addition of 500 無 of water to each of these six samples, they were analyzed as described in 2.3.
2.7. Biliary excretion of (photo)metabolites from NIF
The iliac vena and bile-duct of two prepared rats were cannulated and bile was collected for 10 min; this was used as a blank. The rats were given an intravenous injection of 125 痢 of NIF in 25 無 of PEG+. Immediately thereafter, one rat was kept in the dark and the other exposed to UVA and the bile produced during the following two hours was collected. Without further dilution, the bile of each rat was analyzed as described in 2.3.
2.8. Biliary excretion of metabolites from NONIF
The iliac vena and bile-duct of two prepared rats were cannulated and bile was collected for 10 min; this was used as a blank. The rats were given an intravenous injection of 125 痢 of NONIF in 25 無 of PEG+. Immediately thereafter, the rats were kept in the dark and the bile produced during the following two hours was collected. Without further dilution, the bile of each rat was analyzed as described in 2.3.
2.9. (Photo)reactivity of NIF and photoproducts with BSA
A 10 mg/mL stock solution of NIF, NONIF in ethanol or NHNIF in DMSO was made. Of each of these stock solutions, 50 mL was mixed with 10 mL of BSA in 0.1 M phosphate buffer 7.4. Three aliquots (1.5 mL) of each of these solutions were exposed to UVA for 10 min as described in 2.4. and another three were kept in the dark. Samples were analyzed with HPLC (see 2.3.).
3. Results and discussion
As was previously found [8], the lactam derivative of NIF (NHNIF) proves to be the end-product of the photoreaction in the presence of GSH. The nitroso derivative of NIF (NONIF), which is quantitatively formed in phosphate buffer without GSH, is only an intermediate in that case. UVA-exposure of NIF in plasma and in blood in vitro, also resulted in one detectable product: NHNIF [8].
This was the reason that the investigation of the photoreactivity of NIF in vivo was at first focused on the formation of NHNIF.
Fig.2 shows that after intravenous administration, NHNIF is rapidly cleared from the blood and almost quantitatively excreted via the bile. From these data, it was concluded that collecting the bile for two hours would be sufficient to recover all NHNIF formed after intravenous administration of either NONIF or NIF (the latter in combination with UVA).
In the HPLC chromatogram of the extract of the bile collected during two hours after intravenous administration of NONIF, three extra peaks were observed (Fig. 3, upper panel). These were not found in a blank sample of bile taken before the administration of NONIF. As can be seen in Fig.3, NONIF with a retention time of about 12 min under these conditions [8], is not present in the extract. One of the peaks had the same retention time as NHNIF: 8 min. The fact that this was NHNIF indeed was also confirmed by comparison of its UV absorption spectrum with that of an authen tic sample.
The two other peaks had a UV absorption spectrum similar to that of NHNIF (Fig. 4). These two compounds, photometabolites 1 and 2, showed to be photo-unstable. When the extract of the bile was exposed to UVA for five minutes, the two peaks in the chromatogram disappeared whereas that of NHNIF increased (results not represented). Besides the resemblance of the UV-spectra (Fig. 4), it supports the conclusion that photometabolites 1 and 2 are derivatives of NHNIF.
Intravenous administration of NIF followed by UVA-exposure of the rat for two hours, resulted in a chromatogram of the extract of the bile in which only one extra peak could be observed (Fig. 3; lower panel). This peak was not found without UVA-exposure. It could not be attributed to NIF, because this has a retention time of 15 min under these conditions [8]. The retention time of this compound, its UV-absorption spectrum and photochemical conversion into NHNIF points to photometabolite 1. This is comprehensible, if one takes into account that the photoreaction of NIF proceeds via NONIF as an intermediate.
The occurrence of at least one photoproduct in the bile demonstrates that photometabolites can reach the internal system. Whether biological effects of the photoreactivity of NIF may concern other organs than the UVA-exposed areas depends on the intrinsic activity of photometabolite 1 or of its end-product NHNIF and on its bioavailability.
Beside the toxicity of photoproducts derived from NIF, irreversible binding to biomacromolecules should get attention. The reason for this is that a striking difference has been observed [8] between the photoreaction in water (pH 7.4) which only contains GSH, and that in plasma or blood. With GSH, NHNIF is quantitatively formed whereas in plasma and in blood only traces of NHNIF could be detected [8]. Important with regard to the possible occurrence of irreversible binding in the case of plasma and blood is that while NIF showed to be completely converted, no other photoproduct than a tiny amount of NHNIF could be detected.
Also with the in vivo experiments as described above, the amount of photometabolite 1 (NIF + hn) or that of NHNIF plus photometabolite 1 and 2 (NONIF in the dark) was estimated to be less than 5%. Therefore, we tried to adress the question of irreversible binding by some in vitro experiments:
Samples of BSA with either NIF, NONIF or NHNIF were kept in the dark or exposed to UVA for 10 min, extracted with chloroform and analyzed by HPLC (see 2.9.); the results are represented in Table 1. NHNIF shows to be recovered quantitatively, also after exposure to UVA. Therefore, a contribution to irreversible binding as a result of photoactivation of this compound in vivo is not expected.
UVA-exposure does not have any influence on the mass balance found with NONIF; the difference between samples kept in the dark and those exposed to UVA is not significant.
Although NIF is quantitatively recovered from samples kept in the dark, about 45 % is lost with UVA. There is a striking resemblance between NIF + UVA and NONIF in the dark (or plus UVA) with regard to the percentage of NONIF left after 10 min and that of not recovered material. As without BSA NONIF is the only photoproduct, this resemblance indicates that it is the reactivity of NONIF that leads to about 50% not recovered material.
If BSA is not present, both NIF, NONIF and NHNIF are recovered quantitatively by one extraction with chloroform. This shows that a difference in lipophilicity, if any, between the three compounds is small. The consequence of this is, that a difference between the three compounds with regard to complexation to protein (BSA) is not expected. Therefore, the fact that NONIF is so "poorly extracted" has to be attributed to irreversible binding rather than to complexation with BSA.
Also in these in vitro experiments, it shows that the amount of NHNIF obtained is low; about 1% in both cases: NIF + UVA and NONIF in the dark (or plus UVA). This may give an explanation as well, for the small amount of NHNIF found in vivo. Because of the presence of plasma proteins, it seems not improbable that in vivo too irreversible binding occurs.
With the help of antibodies or the labeled NIF, we hope to verify this tentative conclusion in the next future.
References
[1] G.M.J. Beijersbergen van Henegouwen, Medicinal photochemistry: phototoxic and phototherapeutic aspects of drugs, in B. Testa and U. Meyer (eds.), Advances in Drug Research Vol. 29, Academic Press, New York and London, 1997, 79-170.
[2] D.R. Hartter, The use and importance of nitroaromatic chemicals in the chemical industry, in D.E. Rickert (ed.), Toxicity of Nitroaromatic Compounds, Hemisphere Publishing Corporation, Washington, New York and London, 1984, 1-13.
[3] R.P. Mason and P.D. Josephy, Free radical mechanism of nitroreductase, in D.E. Rickert (ed.), Toxicity of Nitroaromatic Compounds, Hemisphere Publishing Corporation, Washington, New York and London, 1984, 121-140.
[4] C.D. Klaassen and D.L. Eaton, Principles of toxicology, in M.O. Amdur, J. Doull and C.D. Klaassen (eds.), Casarett and Doull疄 Toxicology, The Basic Science of Poisons, Pergamon Press, New York, 1991, 12-49.
[5] I.G. Sipes and A.J. Gandolfi, Biotransformation of toxicants, in M.O. Amdur, J. Doull and C.D. Klaassen (eds.), Casarett and Doull疄 Toxicology, The Basic Science of Poisons, Pergamon Press, New York, 1991, 88-126.
[6] D.E. Rickert (ed.), Toxicity of Nitroaromatic Compounds, Hemisphere Publishing Corporation, Washington, New York and London, 1984.
[7] G.M.J. Beijersbergen van Henegouwen and E. Havinga, Photoreactions of aromatic compounds. XX. Photosubstitution of nitronaphthalenes in alkaline media. Rec. Trav. Chim. Pays Bas 89 (1970) 907-912.
[8] De Vries, H. and G.M.J. Beijersbergen van Henegouwen, Photodegradation of nifedipine under in vivo-related circumstances. Photochem. Photobiol. 62 (1995) 959-963.
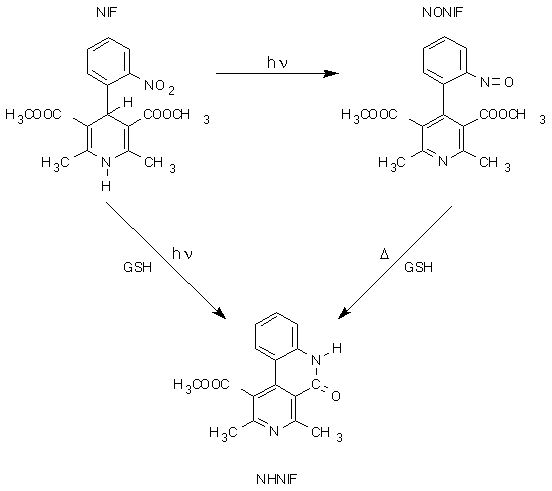
Fig. 1. Photodegradation (hn) of nifedipine (NIF) in 0.01 M phosphate buffer pH 7.4. Without glutathione (GSH) being present, the nitroso derivative NIF (NONIF) is formed and with GSH the lactam derivative (NHNIF); both quantitatively. With GSH being present, NONIF is an intermediate only. This is demonstrated by the thermochemical reaction (D) of pure NONIF with GSH in the same medium, which also quantitatively produces NHNIF.
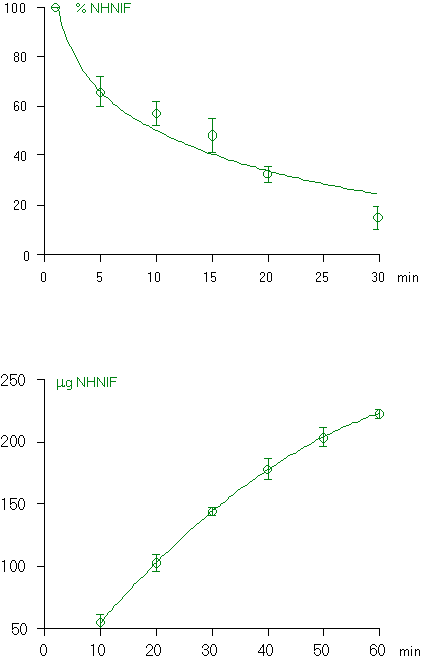
Fig. 2. Clearance of the lactam derivative of NIF (NHNIF) from the blood of rats (upper panel; the concentation at 1 min was put at 100%) and biliary excretion (lower panel) after an intravenous injection of 250 mg NHNIF. Each point is the mean (+SD) of four independent experiments.
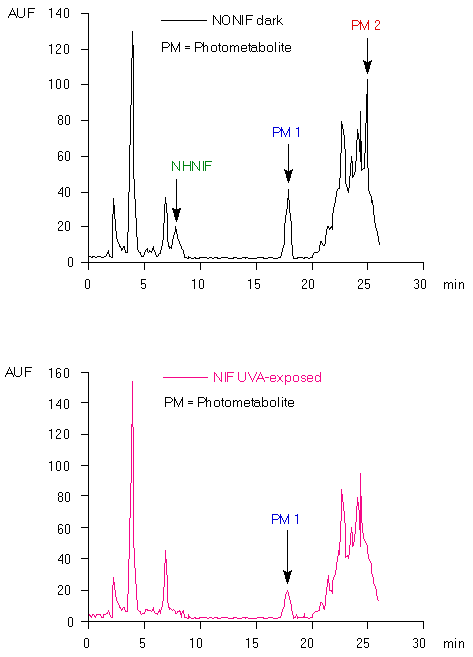
Fig. 3. HPLC chromatogram of the extract of bile, which was collected for two hours immediately after intravenous injection of the nitroso derivative of NIF (NONIF; upper panel). Lower panel: The same was done with nifedipine (NIF); besides, the rat was exposed in that case to UVA for two hours after the administration.
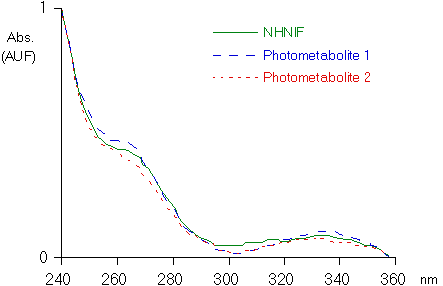
Fig. 4. Ultraviolet absorption spectrum of the lactam derivative of NIF (NHNIF) and of the photometabolite 1 and 2 excreted in the bile of rats (see Fig. 3).
Table 1
(Photo)reactivity of NIF and photoproducts with BSA. Amount of NONIF, NHNIF and not recovered material expressed as percentage (+ SD; n = 3) of starting compound.
NIF |
NONIF |
NHNIF |
Not Recovered |
|
| NIF / UVA | 0 |
54 + 1.8 |
1 + 0.1 |
45 + 1.9 |
| NIF / Dark | 98 + 2.9 |
0 |
0 |
2 + 2.9 |
| NONIF / UVA | --- |
43 + 3.7 |
0.6 + 0.1 |
56 + 3.8 |
| NONIF / Dark | --- |
45 + 2.8 |
0.7 + 0.1 |
54 + 2.9 |
| NHNIF / UVA | --- |
--- |
95 + 5.8 |
5 + 5.8 |
| NHNIF / Dark | --- |
--- |
96 + 6.2 |
4 + 6.2 |