Generation of Superoxide and Hydrogen Peroxide by Exposure of Stilbene-based Fluorescent Whitening Agents to UVA Radiation
 Keith R. Millington
Keith R. Millington
CSIRO Division of Wool Technology,
PO Box 21, Belmont, Victoria 3216, Australia
mailto:keith.millington@dwt.csiro.au
ABSTRACT
We report on the generation of hydrogen peroxide by aqueous solutions of two commercial stilbene-based fluorescent whitening agents (FWAs) in response to irradiation with UV light. A sulphonated distyryl biphenyl FWA (Uvitex NFW) produces both the superoxide anion and H2O2 on exposure to UVA radiation. The formation of superoxide suggests that the excited singlet state of Uvitex NFW undergoes ionisation producing a radical cation and a free electron which is accepted by molecular oxygen. This is consistent with previous flash photolysis work on Uvitex NFW which proposed the formation of transient radical cations. The relationship between the initial concentration of FWA and the amount of H2O2 produced appears complex. The fluorescence excitation spectrum of Uvitex NFW is also strongly dependent on its concentration, but this does not appear be due to the aggregation of FWA molecules.
1. Introduction
Fluorescent whitening agents (FWAs) are important additives which improve the appearance of various commercial products, particularly in the paper, plastics, detergent and textile industries. These latter products usually absorb visible radiation in the blue spectral range of natural sunlight, causing them to appear off-white or yellow. FWAs absorb UVA radiation in the range 360-380 nm and reemit it as visible blue or violet light. Small quantities of FWAs dispersed into these products compensate for the unwanted yellow appearance and also reflect more visible light than was originally incident, making them appear whiter and brighter.
There are three major chemical classes of commercial FWAs, based on the stilbene, coumarin and pyrazoline structures, of which the most widely used are the stilbenes, including distyryl biphenyls, (DSBP). The photochemistry of stilbene-based FWAs has been extensively studied, both in solution and in the solid phase, as stability to sunlight is important in achieving white products with good lightfastness. The presence of oxygen and moisture during irradiation eventually leads to oxidation of the FWA which is then rendered ineffective, causing the substrate to revert to a yellow appearance. Photoisomerisation of the fluorescent trans- isomer to the non-fluorescent cis- isomer is also important, especially in solution [1].
Earlier work on FWA-treated wool fabrics exposed to simulated sunlight in the presence of water has shown that hydrogen peroxide is formed and rapid yellowing of the wool occurs [2]. When wool is doped with before irradiation it yellows even more rapidly. However in the presence of reducing agents such as sodium bisulphite or thiourea dioxide far less yellowing is observed [2]. It was suggested that the generation of H2O2 during exposure to sunlight could be responsible for the increased rate of yellowing of FWA-treated wool, especially when wet, which is a serious commercial shortcoming of the fibre [2].
It was not clear from the earlier work whether the wool protein, the FWA or both were involved in the photochemical mechanism resulting in H2O2 generation, although only wet FWA-treated wool produced measurable amounts of H2O2 after irradiation with simulated sunlight [2]. Certain proteins and their photoproducts, particularly those found in eye lenses such as b-crystallin, have been shown to generate H2O2 and superoxide when exposed to UVA radiation [3,4]. Previous work has shown that irradiation of cadmium sulphide, zinc oxide and metal-free phthalocyanine pigment dispersions can also generate superoxide and H2O2 [5-7]. In this study aqueous solutions of two commercial stilbene-based FWAs of known structure were irradiated using a UVA source in the presence of atmospheric oxygen and analysed for H2O2 and superoxide anion to determine whether FWAs alone can produce these active oxygen species.
2. Experimental
2.1 Materials
Two commercial water soluble FWAs used on wool textiles and having known chemical structures (Uvitex CF and Uvitex NFW) were obtained in concentrated powdered form from Ciba-Geigy AG, Basel, Switzerland. Both were recrystallised twice from aqueous ethanol before use. Their structures are shown below.
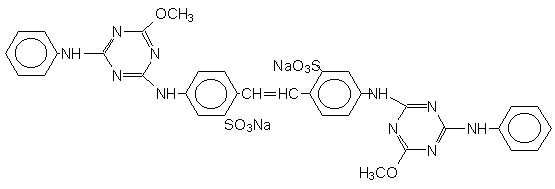
UVITEX CF
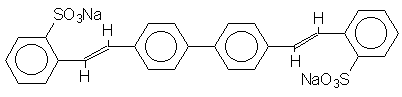
UVITEX NFW
The enzymes catalase (ex bovine liver) and superoxide dismutase (SOD) were obtained from Bohringer Mannheim (Castle Hill, NSW). Ferrous ammonium sulphate, xylenol orange and sorbitol were obtained from Aldrich (Castle Hill, NSW). Hydrogen peroxide (50% w/w) was obtained from Interox (Melbourne, VIC).
2.2 Irradiation conditions
In initial studies aqueous solutions of FWAs were irradiated using a commercial Metrohm 705 UV digester (Metrohm, Switzerland) designed to eliminate moderate amounts of dissolved organic matter from water samples before analysis. The device consists of a water-cooled high-pressure mercury arc surrounded by an annular rack containing twelve quartz sample tubes. The spectral output of the mercury arc covers the whole gamut of wavelengths from UVC to near infrared, and produces a significant amount of heat. Sample temperatures of 70-80oC were obtained after 10 minutes irradiation.
Further studies were carried out using a custom-built UVA irradiation apparatus containing up to twelve Pyrex tubes held in an annular carousel around a central blacklight UVA source (Eye H125BL, Iwasaki Electric Co, Tokyo). This apparatus produced peak output between 360-370 nm and no UV radiation below 300 nm. The lamp was cooled by incorporating a small fan beneath the sample carousel, and the temperature of the solutions during irradiation was kept below 30oC even during extended (up to 6 h) irradiation periods. Radiation intensity was measured using an IL7000 radiometer (International Light, USA) fitted with an SED033/UVA/W detector and filter attachment and calibrated to measure peak irradiance at 360 nm, and was typically 14 mW.cm -2 at the sample position.
2.3 Chemical analyses
Hydrogen peroxide was assayed using the xylenol orange technique described by Jiang et al [8]. H2O2 oxidises iron (II) to iron (III) in the presence of sorbitol, which acts as a catalyst. Iron (III) then forms a purple complex with xylenol orange. A 5 cm3 aliquot of irradiated FWA solution was placed in a 25 cm3 graduated flask, and mixed with 2.5 cm3 each of sorbitol (0.1 M), sulphuric acid (0.25 M) and xylenol orange (1.0 mM). The reaction was initiated by the addition of 2.5 cm3 of ferrous ammonium sulphate (2.5 mM) prepared fresh daily, RO water was added to make up to 25 cm3, and the flask was then shaken for 45 minutes. After shaking the absorbance at 560 nm was determined and compared with a hydrogen peroxide standard curve. The concentration of H2O2 in stock solutions was calculated using the extinction coefficient of 43.6 M-1.cm-1 at 240 nm.
When catalase is added to the FWA aliquot before carrying out the assay, any H2O2 is selectively converted to water and oxygen. As catalase is specific for H2O2,the presence of any other oxidants capable of producing iron (III), including organic hydroperoxides ROOH, can be discriminated.
2.4 Fluorescence spectroscopy
Three-dimensional fluorescence spectra of FWA solutions were obtained using a Hitachi F4500 fluorescence spectrophotometer. Data manipulation and contour plotting were carried out using SAS/Graph software version 6.12 (SAS Institute, Cary, NC).
3. Results
3.1 Production of hydrogen peroxide
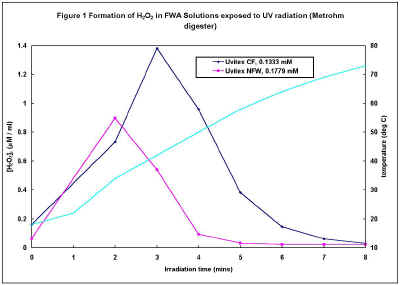
In an initial experiment, aqueous solutions (0.01% w/v) of the two FWAs were irradiated for brief periods up to 10 minutes in the Metrohm UV digester. Immediately following irradiation, aliquots of these solutions were analysed for H2O2 using the xylenol orange technique. Figure 1 shows that both Uvitex CF and NFW produce H2O2 but the concentration decreases after 2-3 minutes irradiation. Figure 1 also shows that the temperature of the solutions increases rapidly through the course of the experiment, and further irradiation experiments using the Metrohm digester were restricted to 2 minutes.
Figure 1 (77k)
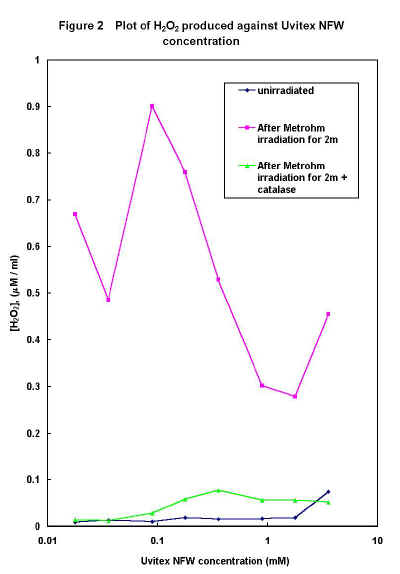
The generation of H2O2 by irradiation of various concentrations of Uvitex NFW for 2 minutes in the Metrohm digester is shown in Figure 2. The variation of H2O2 production with FWA concentration under these conditions appears to be a complex function which is dependent on several variables, likely to include concentration quenching effects and oxygen availability. Figure 2 also shows data for samples to which catalase (~500 units) was added and shaken for 5 minutes before peroxide analysis, which shows little or no H2O2 is present. Since catalase is specific for H2O2, converting it rapidly to water and oxygen, this proves that H2O2 is being generated on photolysis of Uvitex NFW.
Figure 2 (60k)
Having demonstrated the generation of H2O2 from solutions of stilbene-based FWAs using a high-pressure mercury source, it was of interest to investigate the wavelength dependence of the process. For commercial FWAs it is their photochemistry on exposure to sunlight which is important, and in particular exposure to UVA (320-400 nm) which is a more significant fraction of solar spectrum than lower wavelengths and includes the wavelengths responsible for their excitation. Also the high temperatures generated during exposure in the Metrohm digester were undesirable and could cause decomposition of H2O2. Generation of hydroxyl radicals also occurs when H2O2 is exposed to wavelengths below 280 nm - indeed photolytic generation of these radicals followed by their rapid reaction with trace organics is the principle of operation of the Metrohm digester.
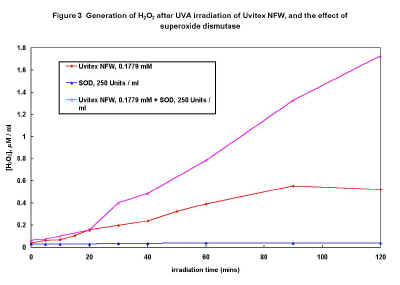
Figure 3
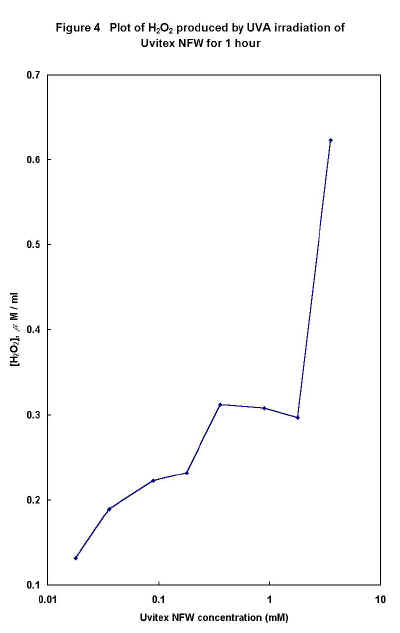
Solutions of Uvitex NFW (0.1779 mM) were exposed in the UVA irradiator for periods up to 2 hours and analysed for H2O2. Figure 3 shows that the generation of peroxide with UVA irradiation time is almost linear up to 90 minutes. Figure 4 shows the effect that variation of FWA concentration has on H2O2 formation after UVA irradiation for 1 hour - again the behaviour appears complex.
Figure 4
3.2 Evidence for involvement of superoxide radical anion
Figure 3 shows the effect of adding SOD (250 Units/ml) to Uvitex NFW solution before irradiation, which results in the generation of significantly higher levels of H2O2 (50% or more) for irradiation periods of 30 minutes or more. UVA irradiation of SOD in the absence of FWA produced no detectable H2O2. Since SOD dismutes the superoxide anion to hydrogen peroxide and molecular oxygen, detection of higher levels of H2O2 in the presence of SOD strongly suggests Uvitex NFW generates superoxide on exposure to UVA radiation.
3.3 Fluorescence of Uvitex NFW
Three-dimensional fluorescence spectra of Uvitex NFW at various concentrations were recorded and demonstrated some unusual behaviour as shown in Figure 5. For Uvitex NFW excitation can occur at several wavelengths depending on the concentration, although the fluorescence emission wavelength remains fairly constant near 430 nm except at very high concentrations. In particular the spectra recorded for concentrations of 35.6 mM and 89 mM show the presence of three excitation maxima, whereas at other concentrations only two excitation wavelengths, characteristic of the aromatic ring (240 nm) and the conjugated double bond (350 nm) chromophores are observed. At very high concentrations (>889.7 mM) excitation at 240 nm becomes ineffective in producing fluorescence.
Figure 5 (large file, 369k)
4. Discussion
The almost linear increase in H2O2 formation with time during UVA irradiation and the increased levels formed in the presence of SOD (Figure 3) suggest that H2O2 is produced by dismutation of the superoxide anion. Superoxide is generated via electron transfer to molecular oxygen, which suggests that photoionisation of the excited singlet state of the FWA probably occurs, ie.
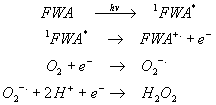
This mechanism is consistent with previous laser flash photolysis work on Uvitex NFW by Ghiggino et al which suggested the formation of semi-oxidised radical cations at 354 nm sample excitation [9]. In view of the results presented here, clearly demonstrating the formation of superoxide and hydrogen peroxide from irradiated Uvitex NFW, the alternative process proposed by Pailthorpe et al suggesting that intersystem crossing of the excited FWA to the triplet state, followed by reaction with ground state oxygen to produce singlet oxygen is considered far less likely, especially for stilbene-based FWAs [10].
The complex fluorescence excitation behaviour observed for Uvitex NFW may well contribute to the non-linear relationship between H2O2 production following UV exposure and FWA concentration expressed in Figures 2 and 4. It was thought that the fluorescence effects might be due to the dimerisation, higher oligomerisation or aggregation of FWA molecules in water. The existence of excimer complexes has also been proposed for stilbene FWAs, but one would expect excimers to show increased emission at longer wavelengths with increasing concentration, but very similar excitation spectra [11].
In previous studies using sulphonated acid dyes of similar molecular weight to the FWAs used here, addition of urea to the dye solution usually causes disaggregation whereas addition of sodium chloride causes an increase in aggregation [12]. Preliminary studies in which urea or NaCl were added to Uvitex NFW solutions had no effect whatsoever on the fluorescence spectrum.
Further work is currently in progress to determine whether superoxide and H2O2 are produced when other commercial FWAs, including those having the pyrazoline and coumarin structures, are irradiated.
5. Conclusions
It has been demonstrated that aqueous solutions of the two commercial stilbene-based FWAs Uvitex CF and Uvitex NFW produce H2O2 when exposed to UV light from a high-pressure mercury arc in the presence of atmospheric oxygen. Uvitex NFW produces both the superoxide anion and H2O2 on exposure to UVA radiation. The formation of superoxide suggests that the excited singlet state of Uvitex NFW undergoes photoionisation producing a radical cation and a free electron which is accepted by molecular oxygen. This is consistent with previous flash photolysis work on Uvitex NFW which proposed the formation of transient semi-oxidised radical cations.
The relationship between the initial concentration of FWA and the amount of H2O2 produced appears complex. The fluorescence excitation spectrum of Uvitex NFW is also strongly dependent on concentration, but this does not appear be due to the aggregation of FWA molecules.
Acknowledgements
Credit is due to Michael Jones for carrying out much of the experimental work and data analysis. The author recognises the support of Australian woolgrowers and the Australian government who fund research and development through the IWS.
References
1. See, for example, Leaver I H and Milligan B, Dyes and Pigments, 5, (1984), 109.
2. Millington K R, Proc 9th Int Wool Text Conf, Biella, Italy, 1995, vol III, 174.
3. Andley U P and Clark B A, Photochem Photobiol, 50, (1989), 97.
4. Linetsky M and Ortwerth B G, Photochem Photobiol, 62, (1995),87.
5. Harbour G R and Hair M L, J Phys Chem, 81, (1977), 1791.
6. Harbour G R and Hair M L, J Phys Chem, 82, (1978), 1397.
7. Freund T and Gomes W P, Catal Rev, 3, (1969), 1.
8. Jiang Z Y, Woollard A C S and Woolf S P, FEBS Lett, 268, (1990), 69.
9. Smit K J and Ghiggino K P, Dyes and Pigments, 13, (1990), 45.
10. Auer P D and Pailthorpe M T, J Photochem Photobiol A: Chem, 86, (1995), 267.
11. See, for example Guilbault G G (ed), "Practical Fluorescence", Marcel Dekker, New York, (1990).
12. Pailthorpe M T in Lewis D M (ed), "Wool Dyeing", Soc Dyers and Colorists, Bradford, (1992), 52.