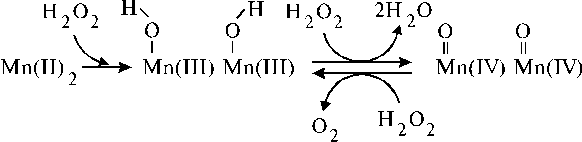
New Approach in Development of Artificial Photosynthetic Systems
for Water Splitting (mini-review)
Alexander V. Udal’tsov
Institute of Biology, Komi Science Center, UrB RAS, Kommunisticheskaya st. 28,
167982 Syktyvkar, Russia,
e-mail: avu@ib.komisc.ru
Contents
I. Introduction
II. Properties of manganese(II) complexes mimicking some characteristics of oxygen-
evolving complex
III. Main problems in design of systems mimicking photosynthetic pathway of water
splitting
IV.Properties of donor-acceptor complexes based on porphyrins
V. Out-of-plane coordination of dimers and associates of porphyrins with transition
metal ions as way for the formation of combined donor-acceptor complexes
VI.Photoinduced self-organization of donor-acceptor complexes as tool for mimicking
of photosynthetic water splitting
VII. Initiation and possible photophysical mechanisms in photosynthetic splitting of water
VIII. Conclusion
Abbreviations: TEA - triethylamine, TPP - meso-tetraphenylporphine, DMF - dimethylformamide, TAPP - meso-tetra(p-aminophenyl)porphine, TDAP - meso-tetra(N,N-dihexadecyl-4-aminophenyl)porphine, PS - photosystem, TTAP - meso-tetra(N,N,N-trihexadecyl-4-aminophenyl)porphine.
I. Introduction
Problems of artificial photosynthesis engage the attention of many researchers in connection with mimicking of solar energy conversion and development of similar way as it takes place in photosynthetic organisms [1-2]. At the present time one of the main problems in this field is the task of water oxidation in artificial photosynthetic systems. It means that the efforts in the study of the processes of artificial photosynthesis are concentrated on system mimicking properties of oxygen-evolving complex (OEC) [3]. There are numerous interesting results in the literature regarding investigation of Mn(II) assemblies, multinuclear manganese complexes or synthetic complexes of pigments involving manganese ions [4-10]. However, this review does not have a goal to consider all approaches and ways in development of the artificial photosynthetic systems for water splitting. The review is dedicated to a round of questions associated with the problems, which are usually not concerned in the literature since the questions are weak studied. The answers to these questions enable us to outline a new approach which is quite perspective from our point of view. Of course, the approach have to be based on relationships similar to those as the photosynthetic apparatus is organized in the living organisms. Hence, main characteristics of an artificial photosynthetic system must be obtained due to non-valence interactions between components of the corresponding model system. In other words, this approach have to be oriented to avoid a complicated synthesis of a model reaction center but with the aim to use technologically simple synthesis, which can be applied for the future high-thin engineering. From this point of view, unusual questions such as the nature of photoactivation and self-organization of donor-acceptor complexes [11-12] or compatibility of different complexes intended for mutual functioning arise.
Now let us have a look at strategic line of photosynthesis mimicking in the history. Initially porphyrin-quinone synthetic models and porphyrin dimers were investigated [13-15], then trimers, strongly coupled dimers and porphyrin arrays [16-19]. And finally complexes of manganese with pigments or synthetic compounds are studied nowadays [20-21]. Special interest of researchers is excited by influence of biomacromolecules (proteins and nucleic acids) on the events of electron transfer [22-23]. From our point of view the most interesting is the interaction between pigment complexes of photosystems I and II, which can provide the key step of biological photosynthesis. Realization of Z-scheme in the reaction center as the events of electron transfer is rather due to the interaction. Properties obtained for a model system, where large-scale aggregates of porphyrin are assembled in ordered structures [24], bring out some evidence for the interaction. Apparently, similar interaction between different pigment systems (chromophores of PS I and II) takes place in biological photosynthesis while the interaction in this case can be promoted by polypeptide chains. Hence, to find the concrete key step triggering the irreversible events of photosynthesis, the goal of the paper has also to include consideration of unusual results obtained earlier under investigation of photosynthetic model systems.
II. Properties of manganese(II) complexes mimicking some characteristics of oxygen-evolving complex
The OEC of PS II contains a manganese cluster consisting of four Mn. Photooxidation of the manganese to a higher valence state occurs for the dioxygen evolving in the photosystem. There are numerous works in the literature mimicking the reactivity of the manganese complex, so that the literature dealing with manganese clusters and complexes produced under the interaction between manganese and dioxygen has been reviewed several times [25-28]. The reader is referred to these articles in order to gain a fundamental understanding of the interactions. Here we present some facts which are interesting from our point of view. The first interesting fact published in the literature is a Mn-Mn dinuclear complex of porphyrin dimer, which was capable to catalyse efficiently the disproportionation of hydrogen peroxide [29]. Another dinuclear manganese(II) complex formed charge-transfer transitions in the corresponding absorption spectrum after addition of excess H2O2 [30]. This dinuclear manganese(II) complex, namely [Mn2(L)(C6H5CO2)2(NCS)], where L = 2,6-bis[N-[2-(dimethylamino)ethyl]imino-methyl]-4-methylphenolate, has been also shown to catalyze H2O2 disproportionation. The possible reaction mechanism, which was described the products observed in the mass spectrum, is partially reproduced in Scheme 1 (we present here only formation of the dimanganese(IV)-oxo complex, while formation of the monomanganese(IV)-oxo complex, in our opinion, can be an intermediate step in this scheme).
Scheme 1
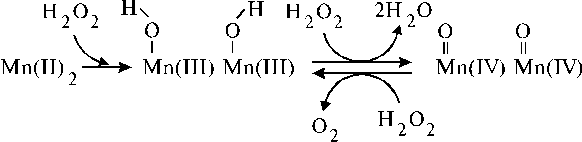
The reacting species have been proposed to be the Mn=O vibration coupled to a ligand-to-metal charge-transfer band [from O2- ® Mn(IV)] through vibronic interaction. It is very important that the dinuclear complex formed the charge-transfer complex under interaction with hydrogen peroxide. Generally speaking, this fact implies simplification for design of model systems mimicking PS I and II since a charge-transfer complex usually has a perfect trapping properties. In this case relationships between the corresponding chromophores can be based on the properties of charge-transfer complexes. These features of the manganese-oxygen interaction can be applied to similar manganese complexes for the design of manganese core of artificial oxidative system.
Design of suitable complex for the study of water oxidation can be perspective when redox chemistry are coupled with acid-base equilibrium of a component of the complex. Similar situation has been found for two Ru(III) centers: [(bpy)2(H2O)RuIIIO-RuIII(H2O)(bpy)2]4+ [31]. This compound possessed a rich redox chemistry coupled with acid-base equilibria of the aqua ligands, so that the redox potentials of the complex were found pH-dependent. Therefore, similar peculiarity of a complex enables a researcher to conduct different redox processes depending on pH.
Results presented in this section evidence that the interaction between manganese and oxygen and redox chemistry can provide advantage for design of model oxidative apparatus based on relevant manganese core. The results accumulated for the present time are quite sufficient for understanding of the corresponding molecular mechanisms. It should be noted that many other interesting results on the same subject are omitted here since we have the other goal of this paper. But at the same time we had to concern this interesting area because manganese core plays main role in the photooxidative processes of photosynthesis.
III. Main problems in design of systems mimicking photosynthetic pathway of water splitting
Photosynthetic pathway of water splitting and design of the corresponding chemical systems still remain complicated problems. These model systems have to operate in aqueous environment similar to that as biological photosynthetic apparatus localized in membrane, is exposed in water environment. There is the other cause for consideration of water environment. It is that only water as a solvent can animate inorganic and organic compounds due to unique properties of liquid water structure making from the compounds living substance of the biological organisms. Our approach suggests mimicking both events of electron transfer in photosynthesis and the corresponding environment also. Generally speaking, in this case design of the chemical systems seems us insoluble problem, since the structure of liquid water remains unknown for the present time nevertheless vast number of models has been suggested for liquid water structure. If we accept the usual way of mimicking, i.e. only those processes and reactions which can be observed in photosynthesis using relevant research techniques, in this case we meet the following problems. The main problems for nowadays include mimicking of oxygen evolving complex, its combination with a suitable pigment apparatus and regulation of trapping of the excitation energy by photosystems I and II. But the problems are not restricted by this round of questions since the general strategic line for the approach remains indistinct. Although there is some strategy, which is laid in development of supramolecular complexes designed on principles of biological complexes from PS II [21].
Our approach is oriented to technologically simple synthesis and therefore we have to consider more detailed chemistry and photochemistry of the corresponding systems. In this respect we can try to represent in the mind how the usual substances are animated by liquid water structure resulting in the living matter. Therefore, our task consists in the investigation of promising photosynthetic model systems so that more information regarding properties of liquid water can be obtained. Hence, we suggest the approach completely different from those for photosynthetic model systems, which are developed by leading laboratory in the world.
First of all let us consider some important results which help us to tune our mind for selection of right direction for future investigation. In this connection, we analyse here results of the work on photoinduced self-organization of a donor-acceptor complex [12]. It was shown that meso-tetra(p-aminophenyl)porphine (TAPP) associate in the presence of TEA-manganese complex, in which manganese(II) has been transformed in the EPR-silent form (perhaps Mn(III)), changed its own structure under steady-state illumination. The transformation of the structure of porphyrin associate occurred in the presence of sodium bicarbonate and was induced by monochromatic light starting with red and finishing ultraviolet light. As a result of the photoreaction initiated by this manner, new (combined) complex consisting of porphyrin dimer coordinated with manganese ions and monomer of the porphyrin, has been formed. According to fluorescence observed under selective excitation, TAPP monomer interacted with the dimer in the excited state, therefore the monomer was involved in the structure of the complex of porphyrin dimer with manganese. If the initial associate of aminoporphyrin (TAPP) was added to the solution of the combined complex after that the steady-state illumination has been completed, in this case the TAPP associate transformed into di-protonated porphyrin dimer. The spectral characteristics of the dimer was very similar to those of di-protonated TPP dimer. Hence, the added aminoporphyrin under these conditions served as an acceptor of protons evolving in the course of the photoreaction. These and other results allow us to propose that some intermediates of water splitting produced under the illumination, bring about the changes of the structure of aminoporphyrin associate and formation of the new complex consisting of manganese and other components.
Hence, the self-organization of donor-acceptor complex (the pigments complex including TEA-manganese complex) takes place as the system was under illumination. In other words, a local electron transfer chain is proposed to be form between components of the system. This photoprocess leading to the formation of new structure of the complex absolutely differs from the reactions of light energy transformation, i.e. the photosynthetic electron transport. Formation or self-organization of the complex under light action occurred as a result of its own unique way of definite reactions. This suggests that there is a pathway of self-organization and evolution of photosynthetic apparatus in plants and higher algae under their growth, when hierarchy of the apparatus is built up gradually in the definite environment. Generally speaking, it means that the mimicking of the photosynthetic apparatus (namely, its main characteristics) is very complicated problem and have to be fulfilled simultaneously with the mimicking of the corresponding environment. Hence, we have to distinguish the photochemical processes leading to the formation of the combined complex and the events of electron transfer resulting in the transformation of light energy. The former processes have a strong dependence on the environment including components to be incorporated in the complex. Meanwhile such model photosynthetic systems as the system investigated in [12] are actually close to biological photosynthetic apparatus because they are designed on the base of non-valence interactions between the components.
Hence, our task consists in thorough analysis of the facts concerning the problem of artificial photosynthesis. Now let us consider relevant experimental results in chronological order since our way of photosynthesis mimicking is based on the facts obtained earlier.
IV. Properties of donor-acceptor complexes based on porphyrins
Strongly-coupled porphyrins demonstrate very fast processes of degradation of the excitation energy [18, 32]. These and other similar strongly-coupled porphyrins mimicking primary photosynthetic events look like perspective for understanding of relationships between porphyrins. Meanwhile porphyrin-quinone compounds are rather modelling some later steps of the corresponding photosynthetic events [33-35]. However, there is no model system displaying clearly a step which would define irreversibility of light energy transformation by photosynthetic organisms. We propose that a photosystem with gradation of rates of electron transfer steps from very fast on the primary step to several order of magnitude slower rate on the late steps has to possess some photophysical or physico-chemical mechanisms preventing loss of excitation energy. In respect of the mechanisms, one way is isomerization or/and protonation of a chromophore in the excited state. But this way is apparently not sufficient to establish the irreversibility of light energy transformation into the energy of separated charges since utilization of water as a resource of electrons and protons is much more complex process than these events. The other way is the cyclic electron transfer conjugated with transport of protons across membrane and accompanied by the appearance of DmH+. This way exists in chloroplasts of living photosynthetic organisms. Regarding model systems, to mimic main steps of photosynthetic events, it is extremely desirable to have a donor-acceptor system with some unique properties which make the system perspective.
First important fact of our investigation is that the charge-transfer complexes have been found in associates of amino derivative of meso-tetraphenylporphine (TPP) in thin films in the presence of water traces [36]. Figure 1 shows absorption spectra of meso-tetra(N,N-dihexadecyl-4-aminophenyl)porphine (TDAP) in the thin films prepared from solutions of the porphyrin in CCl4 and dioxane. We can see that the removing of water from dioxane with molecular sieves led to the disappearance of the charge-transfer band with a maximum at ca. 800 nm (nmax » 12500 cm-1, Dn1/2 » 2000 cm-1). The changes were also observed in the corresponding IR spectra (Fig. 2), where the 1740 cm-1 band was increased simultaneously with the increase of the absorption band at 798 nm in the electronic spectra of thin films containing water traces. The most strong 798 nm band was found in the electronic spectrum of a film when the solvent used has been some dried (Table 1). In this case the corresponding 1740 cm-1 band was also the most intense in the IR spectrum (Fig. 2, curve 5). It should be noted that no charge-transfer band in electronic spectra and no band approximately at 1740 cm-1 in the corresponding IR spectra were observed when TEA or CCl4 have been used as a solvent. These substances have electron donor and electron acceptor affinity relative to porphyrins, respectively.
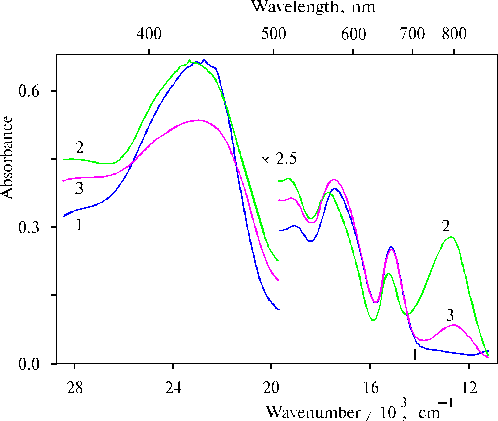
Fig. 1. Absorption spectra of meso-tetra(N,N-dihexadecyl-4-aminophenyl)porphine (TDAP) in thin films prepared by evaporation of porphyrin in: CCl4, 1; and dioxane after drying with molecular sieves for 3 days, 2; and for two weeks, 3. Molecular sieves were replaced after each 3-days staying. Adapted from [36].
Fig. 2. Infrared spectra of TDAP in thin films prepared by evaporation of porphyrin in: triethylamine, 1; CCl4, 2; and dioxane after drying for 2 weeks, 3; without any drying, 4; and after drying for 3 days, 5. Adapted from [36].
Table 1
Ratio of extinction coefficients (ei / e1) of the bands formed by the corresponding electron transitions in the spectra of TDAP associated in thin films.
|
ei / e1a |
Qy(1,0) |
Qx(0,0) |
Charge-transfer band b |
|
e2 / e1 |
0.95 |
0.96 |
- |
|
e3 / e1 |
1.1 |
0.92 |
0.20 |
|
e4 / e1 |
1.2 |
0.90 |
0.59 |
|
e5 / e1 |
1.3 |
0.76 |
0.74 |
a - i is the number of the spectrum, the numeration is similar to that in the Fig. 2.
b - in the case of charge-transfer band, the corresponding extinction coefficient (ei) was related to the coefficient of the band formed by Qy(0,0) electron transition in the spectrum of thin film prepared by evaporation of TDAP c in triethylamine.
c - amount of porphyrin in thin films was estimated from absorption spectra using extinction coefficients obtained for associated meso-tetra(p-aminophenyl)porphine (TAPP) [37].
The above results prompted us to investigate properties of TPP aminoderivatives in details [38]. As the broad Soret bands in the following absorption spectra show, TAPP in solution and TAPP covalently bound to a hydrophobic-hydrophilic copolymer were strongly associated as compared to TPP, which was present in monomeric state in solution since its phenyl rings prevented the dimerization (Fig. 3). The spectra of the associated aminoporphyrins in the region of qausi-allowed electron transitions were also considerably changed as compared with that of TPP. Fluorescence spectra of the associated aminoporphyrins also strongly differed from the spectrum of monomeric TPP (Fig. 4) and exhibited near infrared emission as a long tail. According to differential spectra, mono-protonated aminoporphyrin associate was the radiative center of the near infrared emission (Fig. 5). In contrast, the excitation spectra of TAPP in solution measured in the regions of main and infrared emissions were found to be identical. Hence, the interaction between carboxyl groups and aminoporphyrin molecules bound to the copolymer took place, so that the protonation of the associated aminoporphyrin occurred in the excited state. A new broad band of medium intensity at 1750 cm-1 was found in resonance Raman spectrum of TAPP bound to the copolymer on excitation at 441.6 nm (Fig. 6) [38]. This band was analogous to the 1748 cm-1 band observed in the IR spectra of TDAP associated in thin films in the presence of water traces, when the same solvent (DMF) has been used for the preparation of the film. The both bands indicated the formation of donor-acceptor complex between associated aminoporphyrin molecules involving water in the complex. But in the case of the solution of TAPP bound to the copolymer, the charge-transfer complex was apparently formed in the excited state. Possible structures, which can be precursor of the charge-transfer complex in dimer or associate of aminoporphyrin bound to copolymer, interacting with macromolecular carboxyl groups have been suggested [11].
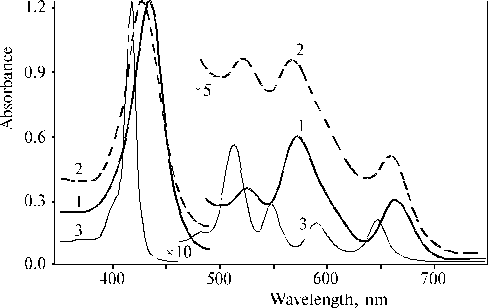
Fig. 3. Absorption spectra of porphyrins in dimethylformamide (DMF): TAPP, 1; TAPP bound to copolymer III (see the composition of the copolymer in Fig. 9), 2; and TPP, 3. Reproduced from [38], Copyright (1993), with permission from Elsevier Science.
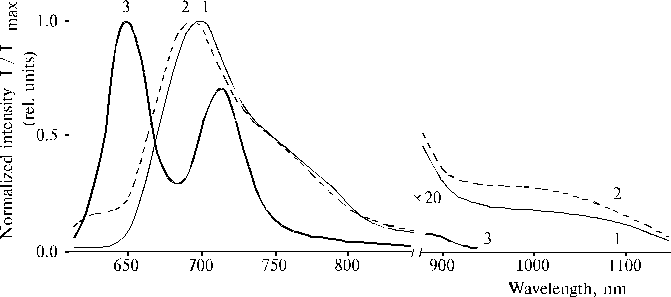
Fig. 4. Fluorescence spectra of porphyrins in DMF: TAPP, 1; TAPP bound to the copolymer III (see Fig. 9), 2; and TPP, 3. Spectra are normalized on the intensity of main emission band. Reproduced from [38], Copyright (1993), with permission from Elsevier Science.
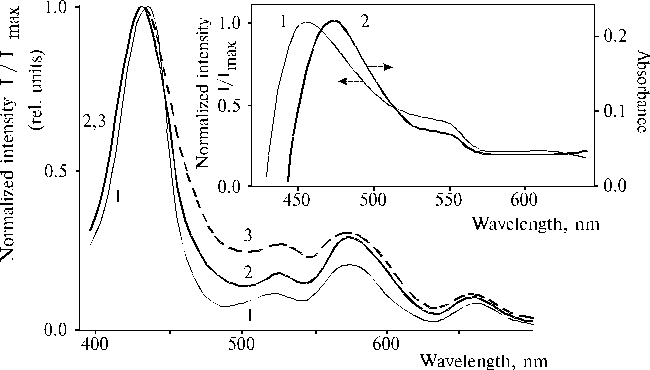
Fig. 5. Excitation spectra of TAPP (1) and TAPP bound to the copolymer III (2, 3) in DMF with registration of emission at 710 nm (2), and 980 nm (1, 3). The inset shows differential spectra of TAPP bound to the copolymer III: the differential spectrum of fluorescence excitation (normalized intensity at lreg. = 980 nm minus normalized intensity at lreg. = 710 nm), 1; and the differential spectrum of absorption of mono-protonated TAPP associate of the same sample and its neutral species, 2. Reproduced from [38], Copyright (1993), with permission from Elsevier Science.
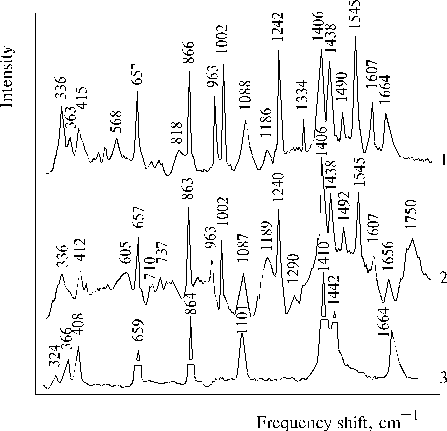
Fig. 6. Resonance Raman spectra of porphyrins in DMF on excitation at 441.6 nm: TAPP, 1; TAPP bound to copolymer III (see Fig. 9), 2; and Raman spectrum of DMF containing water traces, 3. Reproduced from [38], Copyright (1993), with permission from Elsevier Science.
Hence, the finding of donor-acceptor complexes usually realizing as charge-transfer complex under association of aminoporphyrins in thin films in the presence of water traces or covalent binding of aminoporphyrin to hydrophobic-hydrophilic copolymer attracted our attention with the aim of development of chemical model systems for mimicking some steps of photosynthesis.
V. Out-of-plane coordination of dimers and associates of porphyrins with transition metal ions as way for the formation of combined donor-acceptor complexes
Involvement of water in the structure of donor-acceptor complexes formed in thin films of associated aminoporphyrins has been shown for TAPP and TDAP [39-40]. The H-O-H bending vibrations were used for diagnostics of water-porphyrin interactions. In particular, the value of a shoulder at 1653 cm-1 belonging the vibrations of water had a correlation with the peak at 1618 cm-1. The latter frequency is usually associated with proton-donating water molecules [41]. In the case of TAPP in thin films, a shoulder or a broad band with lmax = 760 nm (13150 cm-1) was observed in the electronic spectra of the corresponding films containing water traces, Fig. 7. Hydration of the thin film resulted in an increase of absorption in the 670-800 nm region with lmax = 750 nm (~ 13300 cm-1). In this case IR spectra were also some changed. Formation of the donor-acceptor complex in porphyrin associates was accompanied by appearance of the 1618 cm-1 peak, which however diminished in the IR spectra simultenously with a decrease of water amount in thin films. Hence, water was directly involved in donor-acceptor interaction with aminoporphyrins associated in thin films. These results provide necessary basis for the further investigation of coordination properties of associated aminoporphyrins.
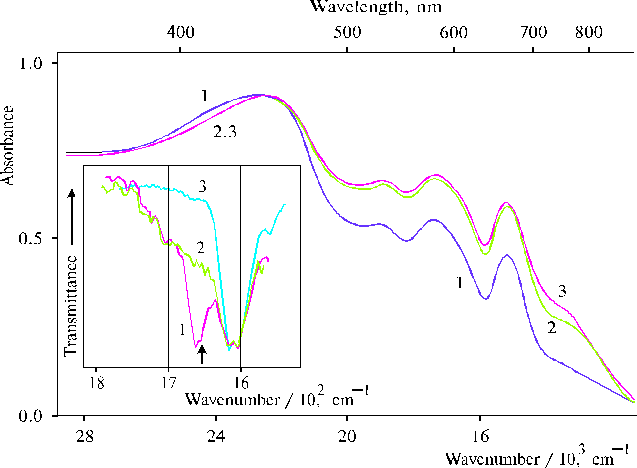
Fig. 7. Absorption spectra of TAPP in thin films prepared by evaporation of porphyrin in DMF in the absence, 1; in the presence of minor amounts of water, 2; and after hydration of the latter, 3. The inset shows IR spectra of TAPP in thin films (1, 2) and in KBr, 3: in the presence of minor amounts of water, 1; and after hydration of the film, 2. Reproduced from [39], Copyright (1996), with permission from Elsevier Science.
Macrocyclic compounds, porphyrins and phthalocyanines, under interaction with metal ions are capable to the formation of metallocomplexes [42]. These reactions have been well studied so that our attention was attracted by other features of porphyrins, which were weak studied or completely not investigated. First of all, the question was whether dimers and associates of porphyrin had a specificity under interaction with transition metal ions. This question excited our interest since porphyrin dimers investigated earlier, had mono-protonated state with partially saturated coordination under interaction with neighbouring porphyrin molecule in the dimer involving water molecules [43].
The study of coordination of manganese(II) ions with protonated TPP dimers demonstrated some interesting features. It was proved that the only one TPP dimer with the maximum of the Soret band at 465 nm interacted with Mn2+ resulting in the formation of the complex [44]. A decrease of the 465 nm band and appearance of a shoulder at 480 nm indicated formation of the complex between this TPP dimer and Mn2+ (Fig. 8). In contrast, interaction of Mn2+ with associated TAPP bound to a copolymer was not displayed so clearly in the electronic spectra. Such behaviour of the spectra is rather due to donor-acceptor interactions in associates of TAPP, where the interactions are very strong so that the characteristic electronic structure of the quasi-allowed transitions keeps its own peculiarity [38] (see Fig. 3). But in the case of the protonated TPP dimers donor-acceptor interactions are quite weak, since the interactions are induced under coordination of proton and subsequent coordination of neighbouring porphyrin molecule in the dimer via two water molecules. That is why the interaction of Mn2+ with the TPP dimer is clear displayed in the spectrum, but in the case of TAPP the stronger interaction between porphyrins retains their characteristics nevertheless of the interaction with Mn2+. Addition of FeCl3 to solution of TAPP bound to copolymer II (see the composition of the copolymer in Fig. 9) in the presence of MnCl2 in the solution led to the appearance of new broad band with a maximum at 760 nm possessing a high extinction coefficient (Fig. 10) [44]. It should be noted that the rest structure in the range of qausi-allowed electron transitions of the porphyrin associate is not almost changed according to the spectra. Hence, the transition metal ions are coordinated with porphyrin dimers (or associates) without incorporation of the ions into the macrocycles. The constant of the complex formation with the out-of plane coordination of the ions was estimated by a way like the binding constants, since the value of absorption of the broad band at 760 nm has been depended from the succession of FeCl3 and MnCl2 addition to solution of TAPP bound to the copolymer II. The higher value of the band was found in the case of the FeCl3 addition after MnCl2, i.e. when the complex between Mn2+ and associated porphyrin has been formed. It was unusual behaviour of the system under formation of the complex so that the applying of the dissociation constant for the characterization would be wrong.
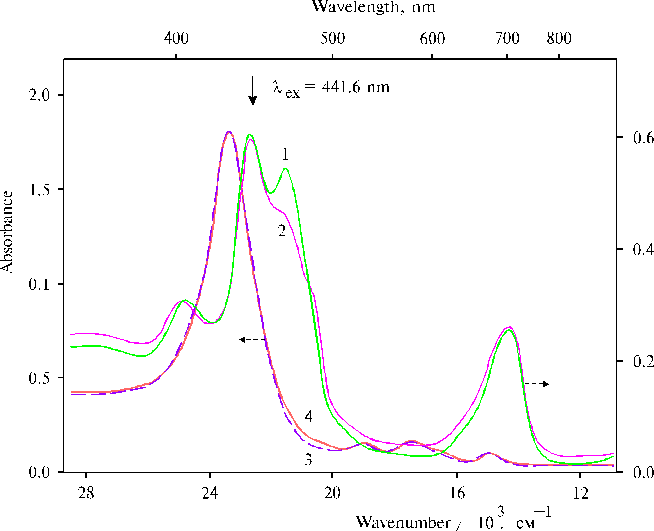
Fig. 8. Absorption spectra of TPP in water : acetone : dioxane (90:5:5, v/v) in the presence of 0.4 N hydrochloric acid, 1; and in the presence of 1.7 ´ 10–3 mol l-1 MnCl2, 2; and TAPP bound to copolymer II (see Fig. 9) in DMF, 3; and in the presence of 1.2 ´ 10–3 mol l-1 MnCl2, 4. Adapted from [44].
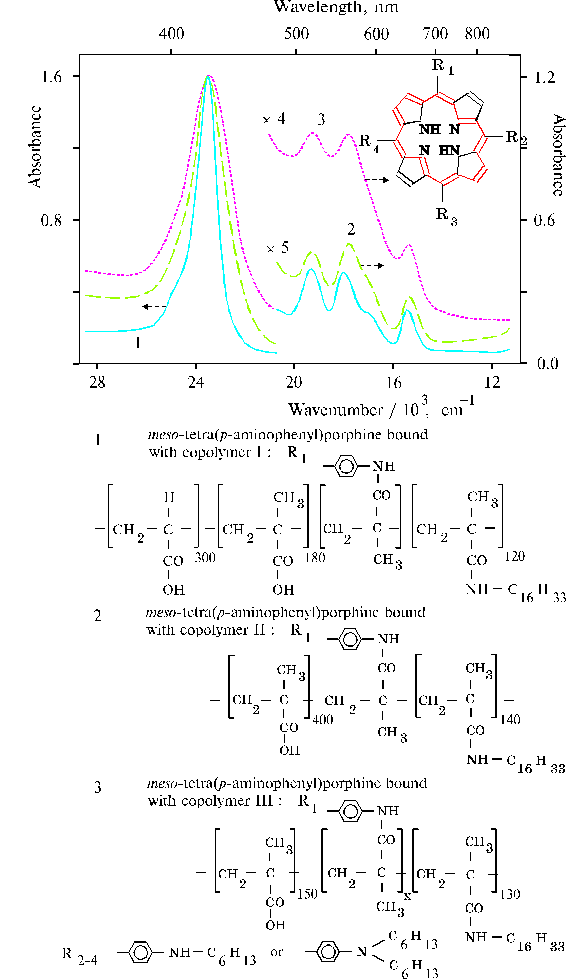
Fig. 9. Absorption spectra of TAPP bound to hydrophobic-hydrophilic copolymers in DMF with different degree of association of the porphyrin: copolymer I, 1; copolymer II, 2; copolymer III, 3; composition of the copolymers is displayed in this figure below.
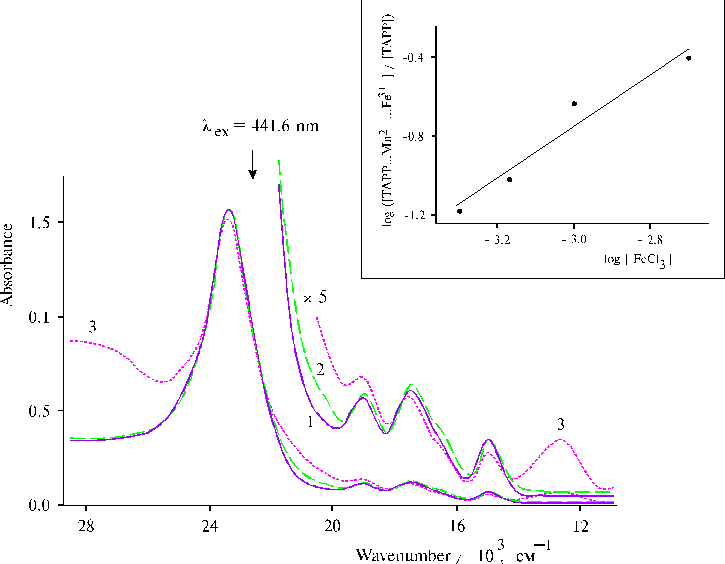
Fig. 10. Absorption spectra of TAPP bound to copolymer II in DMF containing 1-2% of water, 1; in the presence of 1.2 ´ 10–3 mol l-1 MnCl2, 2; in the presence of 1.2 ´ 10–3 mol l-1 MnCl2 and 0.7 ´ 10–3 mol l-1 FeCl3, 3; (optical pathlength is 0.2 cm). The inset shows the plot of log ([(TAPP…Mn2+)…Fe3+] / [TAPP]) vs. log [FeCl3] at the constant value of MnCl2 concentration equaled 5.0 ´ 10–4 mol l-1. Reproduced from [45], Copyright (2000), with permission from Elsevier Science.
Unusual properties of these complexes between Mn2+, Fe3+ and TAPP bound to the copolymer II, namely, some characteristics of absorption, fluorescence and resonance Raman spectra, were found different at different concentrations of porphyrin. The near infrared emission at 840 nm was observed in the fluorescence spectra at a low concentration of TAPP bound to the copolymer (~ 0.6 ´ 10-5 mol l-1). While the infrared emission was almost absent in the spectrum at about four-fold increase of the porphyrin concentration, when the 760 nm band in the electronic spectra was significantly higher in magnitude than the band at the low concentration [45]. At the same time at the low concentration of porphyrin only weak 1810 cm-1 band was observed in a high-frequency region of the resonance Raman spectrum, while strong 1810 ± 3 cm-1 and broad and strong 1910 cm-1 bands were revealed in the spectra at the higher concentration of porphyrin (Fig. 11). These results demonstrate that unusual relationships between components in the combined complexes formed by Mn2+, Fe3+ and TAPP bound to copolymer II takes place, so that the deactivation of excitation by radiative way competes with the vibrational deactivation of the complex or with a reaction producing intermediates, which are responsible for the high-frequency bands in the resonance Raman spectra. It should be noted that according to the spectrum (Fig. 11, curve 2), formation of the complex between TAPP bound to copolymer II and Mn2+ took place after addition of MnCl2, nevertheless the corresponding absorption spectra (Fig. 8, curves 3 and 4) were almost not changed.
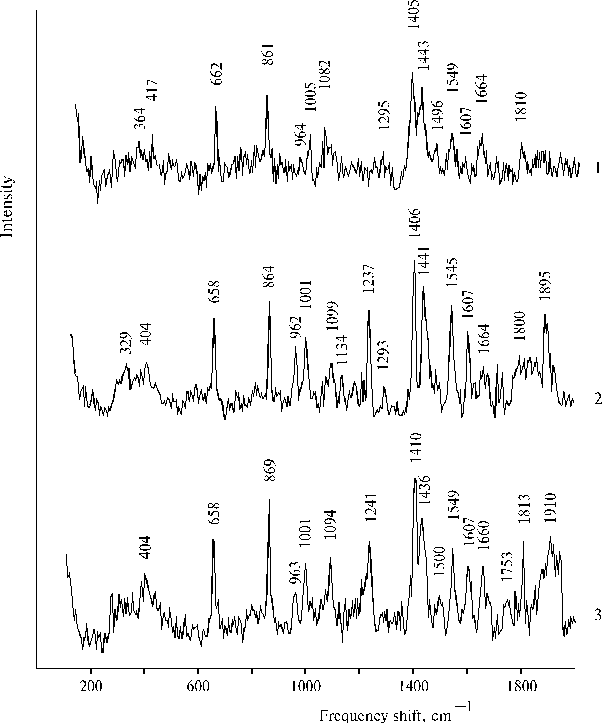
Fig. 11. Raman spectra (lex = 441.6 nm) of TAPP bound to copolymer II in DMF containing 1 - 2 % of water at a low concentration of porphyrin (~ 0.6 ´ 10–5 mol l-1) in the presence of 2.0 ´ 10–3 mol l-1 MnCl2 and 2.0 ´ 10–3 mol l-1 FeCl3, 1; at about four-fold concentration of porphyrin (~ 2.6 ´ 10–5 mol l-1) in the presence of 1.2 ´ 10–3 mol l-1 MnCl2, 2; and in the presence of 1.2 ´ 10–3 mol l-1 MnCl2 and 0.7 ´ 10–3 mol l-1 FeCl3, 3. Reproduced from [45], Copyright (2000), with permission from Elsevier Science.
The concentration dependence of the properties presented above suggests that Mn2+ and Fe3+ are coordinated with different dimers (or associates) of the aminoporphyrin so that the different species of dimeric porphyrin apparently form the complex under out-of-plane coordination of only one ion, Mn2+ or Fe3+. Manganese(II) ions were found to be present in assembled state in the structure of a porphyrin-bound hydrophobic-hydrophilic copolymer [44]. Selectivity of coordination of Mn2+ found for the mono-protonated TPP dimer and assembled state of Mn2+ in biphilic copolymer provide basis for the formation of self-ordering complexes. Hence, in the case of TAPP bound to copolymer II, there are apparently two interacting complexes, one of them is porphyrin dimer coordinated with Fe3+ while the other porphyrin dimer (or associate) is coordinated with assembled Mn2+. From this point of view, the quenching of the near infrared emission of the complex at 840 nm can be interpreted in terms of electron transfer reaction and transition of Fe(III) in the reduced (high-spin) state, Fe(II). In this case the high-frequency bands observed in the resonance Raman spectra are due to generation of some intermediates of the photoreaction.
Hence, polymer-bound aminoporphyrins are suitable pigment systems for design of model complexes and photoreactions mimicking some steps of photosynthesis. These systems, where donor-acceptor interactions are realized in associates of the aminoporphyrin, reveal unusual coordination properties.
VI. Photoinduced self-organization of donor-acceptor complexes as tool for mimicking of photosynthetic water splitting
When biological photosynthetic apparatus is functioning under light action it is present in an organized form. But the organization of the photosynthetic apparatus apparently exists on the level of pigment-protein complexes. The conclusion about the absence of united functioning structural chain of electron transport in membranes of chloroplasts allows to suggest that the photosynthetic electron-transport complexes are organized in assemblies [46]. Namely, the organized electron-transport complex on each step of electron transfer in photosynthesis represents itself as a structural unit. We became substantial simplification of our task accepting similar approach for artificial photosynthesis. Main problem for the present time is oxidative splitting of water mimicking photosynthetic pathway. In general, at least two different complexes with the different functions are needed for mimicking of the steps of oxidative processes. One of them have to be based on a porphyrinic pigment while the other has to involve assembled manganese. In our study the former was the complex formed in associates of aminoporphyrins involving water in the structure of the complex, whereas the other complex with required properties was found under interaction of aliphatic amine with manganese(II) ions [12]. The goal of that study was to look for the possible photoreactions in the case of illumination of the solution starting with red light since donor-acceptor complex formed between water and associated aminoporphyrin absorbs in the red region of the spectral range.
The finding of this study is photoinduced reordering of the structure of the initial complexes (Fig. 12). Although the photoprocesses taking place under the illumination in the presence of bicarbonate ions are still not investigated in details, but the changes observed in the absorption spectra indicate the formation of the complex between manganese ions and TAPP dimer with maxima of the bands at 467 and 778 nm. Characteristics of the spectrum of the complex suggest out-of-plane coordination of manganese ions with porphyrin macrocycle revealed recently. A simple test, namely, addition of the same aminoporphyrin to the illuminated system of two complexes displayed the formation of the other complex, where all added aminoporphyrin was found in the solution as the dimer in the di-protonated state. The changes in the absorption spectra in both cases were interpreted in terms of self-organization of the complexes under the illumination, while the resultant complex was thought to be acceptable for mimicking of water splitting.
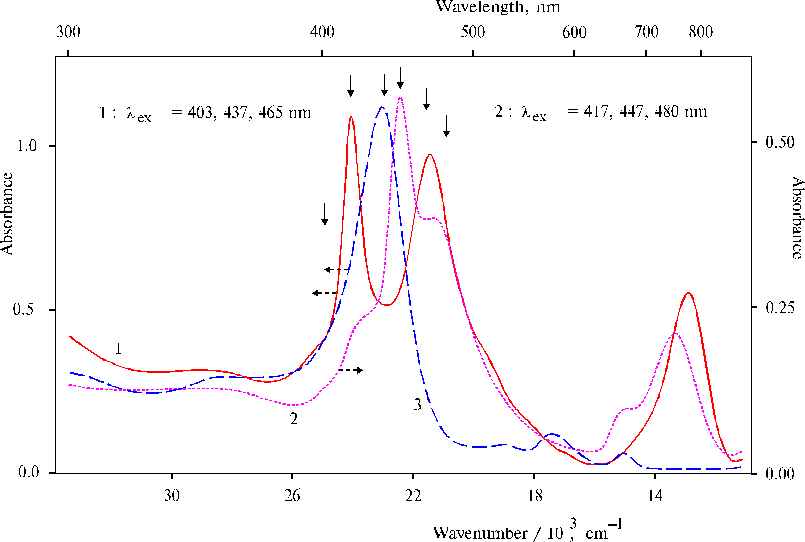
Fig. 12. Absorption spectra of the complex between TAPP and EPR-silent manganese-amine complex in DMF containing 1-2% (v/v) of water preliminary treated with NaHCO3 after 20-min illumination with a non-stop change of the wavelength starting by red and finishing by near ultraviolet light, 1; the mixture of the same solution of the illuminated porphyrin with EPR-silent manganese-amine complex and initial (not illuminated) solution of TAPP, 2 (see details in the text); and TAPP in DMF containing 1-2% (v/v) of water, 3. The points of excitation are marked by the solid arrow. Reproduced from [12], Copyright (2000), with permission from Elsevier Science.
Fluorescence spectra of the complexes exhibited very interesting features, the spectra of one from the complexes are presented in Fig. 13. The strong near infrared emission of the complex on excitation at 465 nm demonstrates efficient involving of the porphyrin dimer-manganese complex in the photoprocess (reader is referred for the details to the paper [12]). On the other hand, this infrared emission can be useful for the study of further transformation of intermediates produced by the photoreaction. These results give us necessary basis for development of self-organizing artificial photosynthetic systems. But more important thing is that the self-organization also suggests a crucial change in the reactive capacity of usually inert water molecules, so that water intermediates produced by this photoprocess perhaps can serve as the promoting agents in the events of irreversible water splitting in artificial systems.
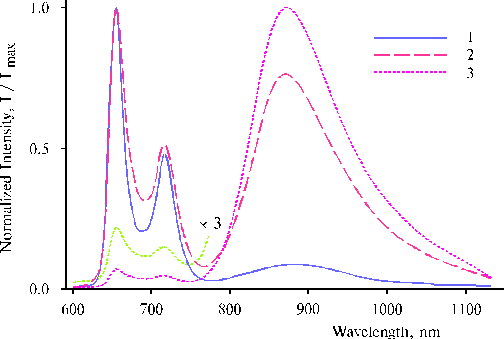
Fig. 13. Fluorescence spectra of the complex between TAPP and the EPR-silent manganese-amine complex formed after the definite illumination on excitation at 403 nm, 1; 437 nm, 2; and 465 nm, 3. The spectra are normalized on the intensity of main emission band. The experimental conditions are the same as indicated in the caption of Fig. 12, 1. Reproduced from [12], Copyright (2000), with permission from Elsevier Science.
Hence, these results open new approach for design of artificial photosynthetic systems intended for solar energy conversion and give us real tool for mimicking of biological photosynthesis.
VII. Initiation and possible photophysical mechanisms of photosynthetic splitting of water
There are many questions regarding water oxidation in PS II and remaining unknown for the present time nevertheless extensive investigation of the photooxidation. In this respect a relevant model system can give an opportunity to study steps of the complicated processes. An irreversible photoreaction in associates of meso-tetra(N,N,N-trihexadecyl-4-aminophenyl)porphine (TTAP) in water containing small amount of water-soluble organic solvent has been revealed recently [47]. This model system demonstrates definite relationships between aminoporphyrin molecules in its associates in water environment and an electron transfer photoreaction, which was interpreted in terms of initial steps of water splitting. We propose that the irreversibility of the photoreaction can provide the driven force for the oxidative process and this peculiarity is most probably based on water-porphyrin interactions in dimers or associates of the aminoporphyrin. Figure 14 shows photoinduced changes in absorption spectra of TTAP under steady-state illumination. These changes remained irreversible for a long time in the solution, but in the electrochemical cell with a chlorine-argentum electrode this photoreaction had the other behaviour (in particular, there was no similar irreversibility) and was accompanied by an increase of acidity of the solution (see details in [47]). In the case of the other solvent (DMF), unusual characteristics in resonance Raman spectra of TTAP on excitation at 441.6 nm have been revealed (Fig. 15). The spectra exhibited two very strong and broad 1901 and 1962 cm-1 bands. Similar but weak 1900 cm-1 band was also found in the spectrum of TAPP at a high concentration of porphyrin. However, no similar bands in this region were observed in the corresponding spectra on excitation at 514.5 nm. Interpretation of these results, generally speaking, meets difficulties but it is extremely clear that any interpretation must be in agreement with the photoreaction found previously for the aqueous solutions of TTAP [47]. In our opinion, these unusual high-frequency bands have relation to photoactivation of water in the case of organic solvent, when water apparently form local clusters in the environment of the organic solvent and is involved in donor-acceptor interaction with porphyrin that takes place on selective excitation into porphyrin dimers or associates. But in the case of aqueous solution containing water-soluble organic solvent, next step, namely the photoreaction can occur because other environment. As a result, we can see intermediates of the photoprocess by absorption spectroscopy. Of course, those results are not quite sufficient for complete argumentation of the initial steps of water splitting and further investigations of similar systems are needed.
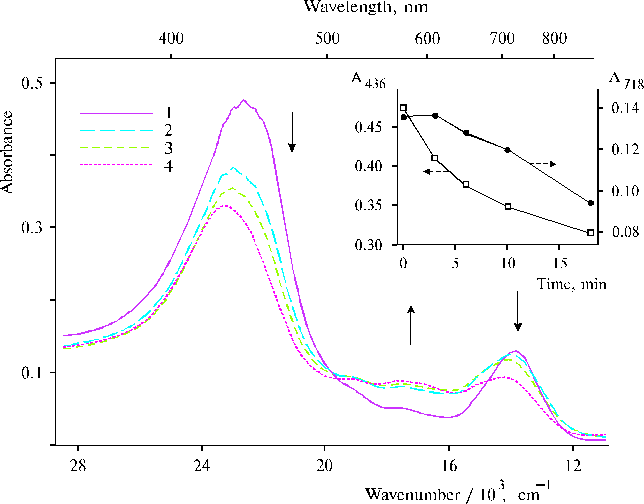
Fig. 14. Absorption spectra of TTAP in water : DMF (95 : 5, v/v) under steady-state illumination by visible light (l > 400 nm): 0, 1; 6, 2; 10, 3; and 17 min, 4. The inset shows kinetics of absorption at the maxima of the bands at 436 and 718 nm. The solid arrows indicate the absorption changes under the illumination. Reproduced from [47], Copyright (1999), with permission from Elsevier Science.
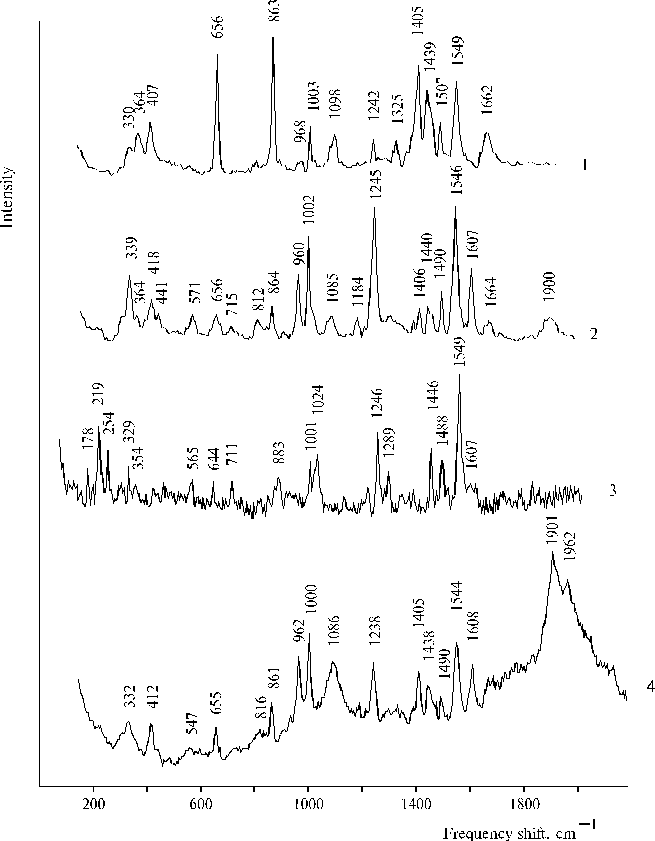
Fig. 15. Resonance Raman spectra of TAPP at a high concentration in DMF containing water traces on excitation at 514.5 nm, 1; and 441.6 nm, 2; TPP in water-glycerol-tetrahydrofuran (86.5 : 10 : 3.5, v/v) in the presence of 0.4 N hydrochloric acid (lex = 441.6 nm), 3; and TTAP in DMF containing water traces (lex = 441.6 nm), 4. Reproduced from [47], Copyright (1999), with permission from Elsevier Science.
To mimic this behaviour in the case of biological system, study of relationships between PS I and II would be preferable under conditions, when very early steps of charge separation can occur in the reaction center but the next steps have to be prevented. However, such situation is almost impossible for the biological system. In contrast, presented here model system (associates of aminoporphyrins) meets the requirements and allows to investigate interaction between different dimeric systems. Now let us pay attention to Z scheme of photosynthesis (all people dealing with photosynthesis are familiar with Z scheme). This scheme provides a definite mechanism in the reaction center to take up electrons from usually inert water, the oxidative potential of water is +1.23 V. This value is covered energetically by the difference between the oxidized state of the lower chlorophyll (P680) and reduced state of the upper chlorophyll (P700) in the scheme. Hence, it is quite naturally to propose a molecular mechanism for the interaction between the porphyrinic pigments of two photosystems. The presence of numerous intermediate electron acceptors in the photosynthetic apparatus, generally speaking, provides rates gradation of electron transfer reactions so that the occurrence of considerably slower late steps are possible in the system. Hence, it is quite possible that some mechanism of interaction between porphyrinic pigments of PS I and II providing direct electron transfer to the upper level and initiating the process exists in the reaction center. Thus, in the case of artificial photosynthesis the following question is arisen. What photophysical mechanisms can be fitted for realization of the interaction between two different porphyrinic systems.
First of all, two porphyrinic systems (at least, two dimers or maybe dimer and monomer interacting with other species, for instance, a complex based on porphyrin) must have the different structures, so that the difference between them on the energy of the ground states would be at least about 0.4 eV (or to be exactly 1.23 V minus oxidative potential of the involved porphyrin of the low-energy system, E(P+./ P). This difference can appear due to the interaction between components of the porphyrin-based systems. Excitation energy for major free-metal porphyrins is not lower than 1.8 eV and therefore the excitation energy exceeds the energy needed for the oxidation of water (+1.23 V). However, fast processes of vibrational relaxation lead to the dissipation of the excitation energy and do not allow the photosystem to accomplish the work of oxidation. To prevent these undesirable processes, a photosystem conjugated with another system energetically saturated (for instance, tetrahedral network of hydrogen bonds of liquid water) is needed. In the case of our model system, interaction between two different water-porphyrin dimeric complexes (mono-protonated TPP dimers of different configurations) can take place since characteristics responsible for involving of water in the structure of the complexes are found. Energy of the H-O-H bending vibrations is close to the energy of exiton interactions in the dimers [48]. When both dimeric complexes are located within the same cooperative structure of liquid water (water cluster), in this case strong interaction involving liquid water structure can be induced due to the proximity of the energies. This photophysical mechanism provides strong interaction between the porphyrin dimers and can cover lack of the energy needed for the oxidation of water. Also it is possible the other mechanism in the system of two different dimers. This mechanism consists in simultaneous transfer of electron and proton in the different porphyrin dimers under photoexcitation. But the realization of this mechanism apparently requires two photons or double portion of the energy needed for water oxidation, i.e. 2.46 eV, since according to the results obtained earlier [47], electron and proton transfers can occur in different dimers with lmax = 403 and 465 nm. It means that only excitation into the Soret band of dimer (or associate) (E* = 2.86 eV for TAPP) can provide realization of this photophysical mechanism. Although, the realization can be provided by strong overlapping red bands of the dimers without the conditions mentioned here.
We presented two hypotheses to explain irreversibility of the process mimicking photosynthesis. In this respect it should be noted that the presence of photophysical mechanism for the interaction between two different porphyrin-based systems allows to design a model photosynthetic system with higher rates of photoreactions not limited by photosynthetic mechanisms of biological photosynthesis.
VIII. Conclusion
The approach for design of chemical model systems outlined in this paper is based on non valence interactions and relationships between compounds and substances, which are participants of complicated processes of water oxidation. Of course, another approach based directly on relationships between elements of PS II [21] has its own advantages. But in our opinion, water can not be a passive substance in these processes. Moreover, irreversibility of electron transfer and water oxidation as a consequence are just due to unique structure and characteristics of liquid water. On the other hand, this approach is only a chain of general insight of self-organizing systems, in which usual substances are animated and organized into living matter due to unique properties of liquid water structure.
References
1 Photosynthesis (translation from Engl.) A.A. Krasnovsky and F.F. Litvin (eds.), Mir, Moscow, 1987, v.1.
2 Energy Resources through Photochemistry and Catalysis, M. Gratzel (ed.), Mir, Moscow 1986 (in Russian).
3 Photosynthetic Oxygen Evolution, H. Metzner (ed.), Academic Press, London, 1978.
4 B.M. Hoffman, C.J. Weschler, F. Basolo, The Dioxygen Adduct of meso-Tetraphenylporphyrinmanganese (II), a Synthetic Oxygen Carrier, J. Am. Chem. Soc. 98 (1976) 5473-5482.
5 Govindjee and R. Khanna, Bicarbonate: its role in photosystem II, in Photosynthetic Oxygen Evolution, H. Metzner (Ed.), Academic Press, London, 1978, pp. 269-282.
6 S.R. Cooper, G.C. Dismukes, M.P. Klein, and M. Calvin, Mixed Valence Interactions in Di-m-oxo Bridged Manganese Comlexes. Electron Paramagnatic Resonance and Magnatic Susceptibility Studies, J. Am. Chem. Soc. 100 (1978) 7248-7252.
7 D.Tang, R. Jankowiak, M. Seibert, C.F. Yocum, G.J. Small, Excited-State Structure and Energy-Transfer Dynamics of Two Different Preparations of the Reaction Center of Photosystem II: A Hole-Burning Study, J. Phys. Chem. 94 (1990) 6519-6522.
8 J. Messinger, J.H.A. Nugent and M.C.W. Evans, Detection of an EPR Multiline Signal for the So* State in Photosystem II, Biochemistry 36 (1997) 11055-11060.
9 K.A. Еhrling, S. Peterson, S. Styring, An Oscillating Manganese Electron Paramagnatic Resonance Signal from the S0 State of the Oxygen Evolving Complex in Photosystem II, Biochemistry 36 (1997) 13148-13152.
10 L. Sun, H. Berglund, R. Davydov, T. Norrby, L. Hammarström, P. Korall, A. Börje, C. Philouze, K. Berg, A. Tran, M. Andersson, G. Stenhagen, J. Märtensson, M. Almgren, S. Styring, and B. Åkermark, Binuclear Ruthenium-Manganese Comlexes as Simple Artificial Models for Photosystem II in Green Plants, J. Am. Chem. Soc. 119 (1997) 6996-7004.
11 A.V. Udal'tsov, Characteristics of donor-acceptor complexes formed in porphyrin-polymer systems and their photoactivation in electron transfer photoreaction J. Photochem. and Photobiol. B: Biol., 37 (1997) 31-39.
12 A.V. Udal'tsov, Yu.V. Kovalev, Photoinduced self-organization of donor-acceptor complex intended for oxidative splitting of water, J. Photochem. and Photobiol. A: Chem., 135 (2000) 193-202.
13 Te-Fu Ho, A.R. McIntosh, J.R. Bolton, Intramolecular photochemical electron transfer in a linked porphyrin-quinone molecule as a model for the primary step of photosynthesis, Nature 286 (1980) 254-256.
14 T.L. Netzel, M.A. Bergkamp, C.-K. Chang, J. Dalton, A comparison of ultrafast electron transfers in porphyrin-quinone and magnesium-free-base diporphyrin molecules: Mimicking photosynthetic charge separations, J. Photochem. 17 (1981) 451-460.
15 N. Mataga, A. Karen, T. Okada, S. Nishitani, N. Kurata, Y. Sakata, S. Misumi, Picosecond Dynamics of Photochemical Electron Transfer in Porphyrin-Quinone Intramolecular Exciplex Systems, J. Phys. Chem. 88 (1984) 5138-5141.
16 O.Bilsel, J.W.Buchler, P.Hammerschmitt, J.Rodriguez, D.Holten, Electronic States and (p, p*) Absorption and Emission Characteristics of Strongly Coupled Porphyrin Dimers: Sandwich Complexes of HfIV and ZrIV, Chem. Phys. Lett. 182 (1991) 415-421.
17 A. Osuka, S. Nakajima, K. Maruyama, N. Mataga, T. Asahi, I. Yamazaki, Y. Nishimura, T. Ohno, K. Nozaki, 1,2-Phenylene-Bridged Diporphyrin Linked with Porphyrin Monomer and Pyromellitimide as a Model for a Photosynthetic Reaction Center: Synthesis and Photoinduced Charge Separation, J. Am. Chem. Soc. 115 (1993) 4577-4589.
18 J.-C. Chambron, A. Harriman, V. Heitz, J.-P. Sauvage, Ultrafast Photoinduced Electron Transfer Between Porphyrinic Subunits Within a Bis(Porphyrin)-Stopered Rataxane, J. Am. Chem. Soc, 115 (1993) 6109-6114.
19 G.M. Dubowchik, A.D. Hamilton, Towards a Synthetic Model of the Structure of the Photosynthetic Reaction Center, J. Chem. Soc., Chem. Commun. (1986) No. 17, 1391-1394.
20 H. Berglund-Baudin, L. Sun, R. Davydov, M. Sundahl, S. Styring, B. Åkermark, M. Almgren, L. Hammarström, Intramolecular Electron Transfer from Manganese (II) Coordinatively Linked to a Photogenerated Ru(II)-Polypyridine Complex: A Kinetic Analysis, J. Phys. Chem. A 102 (1998) 2512-2518.
21 A. Magnuson, Y. Frapart, M. Abrahamsson, O. Horner, B. Åkermark, L. Sun, J.-J. Girerd, L. Hammarström, S. Styring, A Biomimetic Model System for the Water Oxidizing Triad in Photosystem II, J. Am. Chem. Soc. 121 (1999) 89-96.
22 I. Hamachi, S. Tsukiji, S. Shinkai, S. Oishi, Direct Observation of the Ferric-Porphyrin Cation Radical as an Intermediate in the Phototriggered Oxidation of Ferric- to Ferryl-Heme Tethered to Ru(bpy)3 in Reconstituted Myoglobin, J. Am. Chem. Soc. 121 (1999) 5500-5506.
23 S.R. Rajski, S. Kumar, R.J. Roberts, J.K. Barton, Protein-Modulated DNA Electron Transfer, J. Am. Chem. Soc. 121 (1999) 5615-5616.
24 A.V. Udal'tsov, L.A. Kazarin, A.A. Sweshnikov, Self-assembly of large-scale aggregates of porphyrin from its dimers and their absorption and luminescence properties, J. Mol. Struct., 562 (2001) 227-239.
25 W.M. Coleman, L.T. Taylor, Dioxygen Reactivity-Structure Correlations in Manganese(II) Complexes, Coordin. Chem. Rev. 32 (1980) 1-31.
26 V.L. Pecoraro, M.J. Baldwin, A. Gelasco, Interaction of Managese with Dioxygen and Its Reduced Derivatives, Chem. Rev. 94 (1994) 807-826.
27 V.K. Yachandra, K. Sauer, M.P. Klein, Manganese Cluster in Photosynthesis: Where Plants Oxidize Water to Dioxygen, Chem. Rev. 96 (1996) 2927-2950.
28 W. Rüttinger and G.C. Dismukes, Synthetic water-oxidation catalysts for artificial photosynthetic water oxidation, Chem. Rev. 97, (1997) 1-24.
29 Y. Naruta, K. Maruyama, High Oxygen-Evolving Activity of Rigidly Linked Manganese(III) Porphyrin Dimers. A Functional Model of Manganese Catalase, J. Am. Chem. Soc. 113 (1991) 3595-3596.
30 H. Sakiyama, H. Okawa, R. Isobe, A Functional Model of Manganese Catalase. Mass Spectroscopic and Visible Spectral Evidence for {MnIV(=O)}2 and MnII MnIV(=O) Intermediates, J. Chem. Soc., Chem. Commun., (1993) 882-884.
31 J.A. Gilbert, D.S. Eggleston, W.R. Murphy, Jr., D.A. Geselowitz, S.W. Gersten, D.J. Hodgson, T.J. Meyer, Structure and Redox Properties of the Water-Oxidation Catalyst [(bpy)2(OH2)RuORu(OH2) (bpy)2]4+, J. Am. Chem. Soc. 107 (1985) 3855-3864.
32 T.L. Netzel, P. Kroger, C.K. Chang, I. Fujita, J. Fajer, Electron transfer reactions in cofacial diporphyrins, Chem. Phys. Lett. 67 (1979) 223-228.
33 T.L. Netzel, M.A. Bergkamp, C.K. Chang, Benzoquinone Quenching of Diporphyrin Excited States: Kinetic Evidence for Distinguishing Electron-Transfer Photoproducts from (p,p*) States, J. Am. Chem. Soc. 104 (1982) 1952-1957.
34 M.R. Wasielewski, M.P. Niemczyk, W.A. Svec, E.B. Pewitt, High-Quantum-Yield Long-Lived Charge Separation in a Photosynthetic Reaction Center Model, J. Am. Chem. Soc. 107 (1985) 5562-5563.
35 D.G. Johnson, M.P. Niemczyk, D.W. Minsek, G.P. Wiederrecht, W.A. Svec, G.L. Gaines, III, M.R. Wasielewski, Photochemical Electron Transfer in Chlorophyll-Porphyrin-Quinone Triads: the Role of the Porphyrin-Bridging Molecule, J. Am. Chem. Soc. 115 (1993) 5692-5701.
36 A.V. Udal'tsov, V.S. Pshezhetskii, Modelling of Photosynthesis. Charge Transfer Complex Formation in Thin Layer of Porphyrin, Khim. Fizika, 8 (1989) 199-203 (in Russian).
37 A.V. Udal'tsov, V.S. Pshezhetskii, Photoinduced Formation of Dimer Cation of Porphyrin, Khim. Fizika, 7 (1988) 1656-1660 (in Russian).
38 A.V. Udal'tsov, V.Z. Paschenko, A.A. Churin, V.B. Tusov, V.S. Pshezhetskii, Donor-acceptor interactions in porphyrin associates immobilized in biphilic copolymer. J. Photochem. and Photobiol. B: Biol., 21 (1993) 87-94.
39 A.V. Udal'tsov, L.A. Kazarin, Stabilization of Donor-Acceptor Complexes Formed by Associated Porphyrins in Thin Films, J. Photochem. and Photobiol. A: Chem., 96 (1996) 99-107.
40 A.V.Udal'tsov, L.A. Kazarin, Influence of water on spectral properties of porphyrin associates in thin films, Biochemistry (Moscow), 61 (1996) 367-373.
41 G.V. Yukhnevich, Infrared Spectroscopy of Water, Nauka, Moscow, 1973 pp. 117-154 (in Russian).
42 Porphyrins and Metalloporphyrins, K.M. Smith (ed.), Elsevier, Amsterdam, 1975.
43 A.V. Udal'tsov, A.A. Churin, Molecular Complexes Between Dimeric Forms of Porphyrin and Water and their Vibrational Dynamics, Internet Photochem. and Photobiol., (1998) http://www.photobiology.com/IUPAC98/Udaltsov/index.htm
44 A.V. Udal'tsov, A.A. Churin, K.N. Timofeev, Yu.V. Kovalev, Transition Metal Ion Complexes with Associates of Porphyrin Bound to a Hydrophobic-Hydrophilic Copolymer, Biochemistry (Moscow), 64 (1999) 795-802.
45 A.V. Udal'tsov, Yu.V. Kovalev, A.A. Churin, Complexes produced by associated forms of porphyrin bound with hydrophobic-hydrophilic copolymer and transition metal ions, Biophys. Chem., 83 (2000) 141-152.
46 A.B. Rubin, V.P. Shinkarev, Electron transport in biological systems, Nauka, Moscow, 1984 (in Russian).
47 A.V. Udal'tsov, Initial steps of photosynthetic water splitting by associates of porphyrin, J. Photochem. and Photobiol. A: Chem., 130 (1999) 21-33.
48 A.V. Udal'tsov, A.A. Churin, Photochemical Properties of Donor-Acceptor Complexes Formed by Associated Forms of Porphyrin, Internet Photochem. and Photobiol. (1998) http:// www.photobiology.com/v1/udaltsov/udaltsov.htm