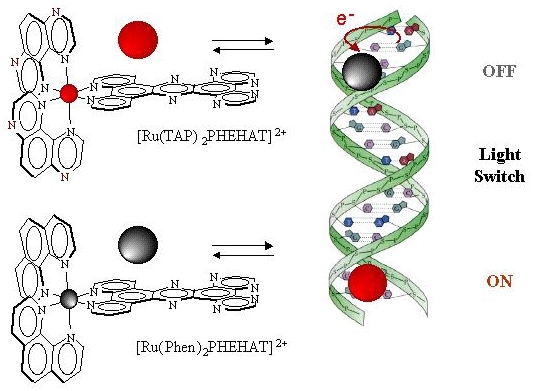
Photoprobes : "Light Switch"

In this scheme two distinct behaviour are presented :
![]() [Ru(TAP)2PHEHAT]2+ is a photo-oxidising complex
(indeed the presence of 2 TAP ligands give the complex a high P*red)
and present a high affinity for DNA (afforded by the intecalating ligand PHEHAT). The luminescence
of this complex is quenched very efficently by DNA and this complex
present thus a "light switch" OFF effect.
[Ru(TAP)2PHEHAT]2+ is a photo-oxidising complex
(indeed the presence of 2 TAP ligands give the complex a high P*red)
and present a high affinity for DNA (afforded by the intecalating ligand PHEHAT). The luminescence
of this complex is quenched very efficently by DNA and this complex
present thus a "light switch" OFF effect.
![]() [Ru(phen)2PHEHAT]2+
present a singular photophysical behaviour. In aqueous solution,
this complex does not emit light at room temperature. Indeed this
is related to the fact that the luminophore in this case is the
Ru-PHEHAT transition which is very effenctly quenched by non-radiative
deactivation throught OH vibrators. In the presence of DNA, the
luminophore is protected from aqueous deactivation as it is tightly
bound to DNA and the complex exhibit intense luminescence. This
"light switch" ON effect is promising in order to detect
eficient photoprobes of the genetic material.
[Ru(phen)2PHEHAT]2+
present a singular photophysical behaviour. In aqueous solution,
this complex does not emit light at room temperature. Indeed this
is related to the fact that the luminophore in this case is the
Ru-PHEHAT transition which is very effenctly quenched by non-radiative
deactivation throught OH vibrators. In the presence of DNA, the
luminophore is protected from aqueous deactivation as it is tightly
bound to DNA and the complex exhibit intense luminescence. This
"light switch" ON effect is promising in order to detect
eficient photoprobes of the genetic material.
It illustrates also how to tune the properties of Ru(II) complexes simply by changing the ligands. Compared to the first complex, the 2 TAP ligands have been replaced by 2 phen ligands which differs only by 2 N.
C. Moucheron, A. Kirsch-De Mesmaeker, J. Physical Organic Chemistry, 1998, 11, 577-583
Structural/Conformational Photoprobes
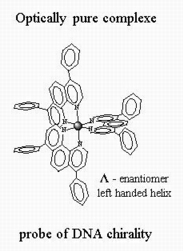
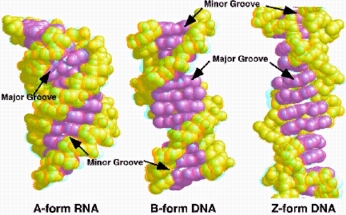
Some Ru(II) complexes have been resolved into their enantiomers D and L (see next section of the introduction for further information) in order to probe potential stereoselective interaction with DNA. Indeed, DNA present different possible secondary structures differing in their helicity (A- and B-DNA right handed helix while Z-DNA present a left handed helix).
For [Ru(DIP)3]2+, a specific interaction of the L (left handed) enantiomer with Z-DNA (also of left handed helicity) has been proposed but this question is still debated. Other optically pure complexes have been studied in the litterature but until now however only weak stereoselectivity has been reported.
C. Kumar, J. Barton and N. Turro, J. Am. Chem Soc, 1985, 107, 5518
H. Kim, P. Lincoln, B. Norden and E. Tuite, J. Chem. Soc., Chem. Comm., 1997, 2375
One of the objective of our work is to synthetise new optically pure complexes in order to test their stereoselectivity of interaction toward DNA.