| Papers and Posters | Site Home Page |
Photodynamic effect of different aluminum and zinc phthalocyanines on isolated nerve cell
A.B.Uzdensky1, A.A. Zhavoronkova1, O.Yu. Dergacheva1, V.M. Derkacheva2
1) Rostov University, Department
of Biophysics, Rostov-on-Don, 344090, Russia.
E-mail: uzd@krinc.ru
2) NIOPIK, Moscow, 103787, Russia
![]()
ABSTRACT
The photodynamic effects of sulphonated zinc (ZnPcS2, ZnPcS3 and ZnPcS4) and aluminum (AlPcS3) phthalocyanines as well as phosphonated aluminum phthalocyanine on the firing of isolated crayfish mechanoreceptor neurons were studied. After 30 min photosensitization neurons were irradiated with He-Ne laser (632,8 nm, 0,3 W/cm2) and changes in neuron firing frequency were recorded. Neuron firing was found to be very sensitive to photodynamic impact and served as a sensitive indicator of cell photodamage. The dynamics of the neuron responses to photodynamic effects included stages of firing activation and/or inhibition prior to irreversible firing abolition and depended on the photosensitizer type and concentration. The comparison of the dependencies of neuron lifetime on photosensitizer concentrations showed that the most effective photosensitizer was ZnPcS2. High photodynamic efficiencies of phthalocyanines was related with the weak dependence of photodynamic effect on sensitizer concentration indicating to the initiation about 3 secondary chain processes such as free radical membrane damage caused by photon absorption by one photosensitizer molecule.
Key words: photodynamic effect, photosensitizers, phthalocyanines, neuron, firing
![]()
1. INTRODUCTION
Photodynamic (PD) therapy is a new promising cancer treatment modality. Red or near infra-red laser light delivered to the stained (photosensitized) tumor excites the dye (photosensitizer) molecule which generates cytotoxic free radical products such as singlet oxygen selectively killing tumor cells. Phthalocyanines are receiving increasing attention as second-generation photosensitizers for PD therapy. Sulphonated Al and Zn phthalocyanines demonstrate favorable physico-chemical and spectral properties for use as photosensitizers (PS)1-3. Recently an isolated crayfish mechanoreceptor neuron (non-traditional for PD researches but informative model object) has been proposed to use in the study of PD effect at the cellular level and PS comparison4,5. Biochemistry, physiology and photobiological responses of this cell are well studied6-8. Its advantage is the ability to fire with a nearly constant rate during several hours. This stable background provides the continuous registration of cell response dynamics from the initial threshold shifts to terminal events leading to the cell death.
The purpose of this paper was to study the dynamics of isolated neuron response to PD effect of a set of aluminum and zinc phthalocyanines in order to compare their PD efficiencies and to obtain information on some mechanisms of PD effect of these PSs at the cellular level.
![]()
2. METHODS
Slowly adapting muscle receptor organs of the crayfish Astacus leptodactilus were isolated as described by Wiersma et al.9. These were placed into a plexiglass chamber with van Harreveld saline (mM: NaCl - 205; KCL -5.4; NaHCO3 - 0.24; MgCl2 - 5.4; CaCl2 - 13.5; pH 7.2-7.4). In this preparation, stretch receptor neurons were capable of regular firing at a nearly constant rate for up to 8-12 hours. Neuron spikes were derived extracellularly from axon by the glass pipette suction electrodes, amplified (amplifier UU-90, IEM, Leningrad, Russia). Their frequency was converted into voltage by the analog frequency meter (MFU-1, IEM, Leningrad, Russia) and continuously recorded by the chart-recorder (N-390, ZIP, Krasnodar, USSR). To test the irreversibility of neuron activity abolition we recorded neuron potentials 30-60 min after cessation of spikes and then additionally stimulated SRN by receptor muscle extension. The absence of spikes indicated that neuron had lost the ability to fire.
The experimental protocol was as follows: at the beginning of each experiment the initial neuron frequency level was set near 10-15 Hz by application of the appropriate receptor muscle extension. After 30 min 'control' recording of spike generation, the PS solution was added into the chamber. After further 30 min, cells were irradiated with the helium-neon laser (632,8 nm, 0.3 W/cm2, LGN-111, "Polyaron", Lvov, USSR,) until the irreversible firing cessation. The irradiation power was measured by the laser dosimeter (IMO-2N, "Etalon", Volgograd, USSR). The irradiation exposures were as long as the neuron lifetimes.
The following photosensitizers synthesized in NIOPIK, Moscow, Russia were studied:
| Photosens: sodium salt of sulphonated aluminum phthalocyanine, AlPcSn , where mean n 3.1; | |
| Phosphonated aluminum phthalocyanine: octa(oxyetoxyphosphinylmethyl) phthalocyanine aluminum hydroxide: HOAlPc [CH2PO(OC2H5)OH]8 , or PAlPc10; | |
| Sodium salts of di-, tri- and tetrasulphonated zinc phthalocyanines: ZnPcSn, where mean n 2.13, 3.06 and 3.74, respectively (ZnPcS2, ZnPcS3 and ZnPcS4). |
Their composition and properties are shown in the Table 1.
All experiments were carried out at a room temperature of 203 oC. Standard statistical methods including correlation and regression analysis11 were used. To compare efficiencies of different photosensitizers we studied concentration dependencies of neuron lifetime (T). Functions T(C) were approximated by the power functions: T(C) = a* Cb, which are linear in the double logarithmic coordinates: lg T= lg a + b* lg C . Parameters a, and b were determined by the least-squares method.
![]()
Table 1. Composition and spectral
characteristics of the studied phthalocyanines
Photosensitizer |
Mean n |
Composition (%) n=1 n=2 n=3 n=4 |
Lmax,nm DMF/HS# |
emax105, l /mol*cm* |
k =emax/e633 |
|||
| AlPcS3 | 3.1 |
- |
15 |
50 |
35 |
677/676 |
2.0 |
6.5** (0.81) |
| ZnPcS2 | 2.1 |
6 |
74 |
13 |
7 |
671/630-675 |
2.14 |
1.0** (0) |
| ZnPcS3 | 3.1 |
- |
22 |
51 |
27 |
675/660 |
1.69 |
1.3** (0.11) |
| ZnPcS4 | 3.7 |
- |
1.5 |
23 |
75.5 |
674/668 |
1.59 |
3.6** (0.56) |
| PAlPc | - |
HOAlPc[CH2PO(OC2H5)OH]8 | 695/691 |
1.27 |
4.5** (0.65) |
|||
# DMF - dimethylformamide; HS - van Harreveld's saline; * - recorded in DMFA; **
recorded in HS.
![]()
3. RESULTS AND DISCUSSION
3.1. Neuron response dynamics
As earlier4,5, the frequency of neuron firing was found to be insensitive to either He-Ne laser irradiation or photosensitization in the dark. However, the combination of these factors (PD effect) caused significant firing changes. Neuron response dynamics included phases of firing acceleration and/or inhibition. Prolonged irradiation caused an irreversible firing cessation that we considered as a functional sign of the cell death. Two main firing abolition processes were observed: (a) firing activation followed by its abrupt abolition, or (b) gradual firing inhibition resulting in the irreversible cessation of spike generation. In both cases firing did not resumed spontaneously or under additional adequate stimulation (receptor muscle extension). The main types (Table 2) of the neuron response dynamics to PD impact (the alternating of firing excitation (E) and inhibition (I) phases) depended on PS type and concentration.
![]()
Table 2. The main types of neuron response
dynamics to PD effect
| Responses | Firing changes |
| E | activation followed by abrupt firing cessation |
| EIE | activation, inhibition, and new activation followed by abrupt firing cessation |
| IE | activation and then acceleration followed by abrupt firing cessation |
| I | gradual inhibition until irreversible firing cessation |
| EI | activation and gradual inhibition until irreversible firing cessation |
| IEI | inhibition, activation, and new gradual inhibition until irreversible firing cessation |
![]()
The percentage of different neuron responses depended on the type and concentration of phthalocyanines (Table 3). In the case of AlPcS3 sensitization the most frequent neuron response was EIE. It was observed at both low and high concentrations in 58 and 78 % of the experiments, respectively. Response E was the most typical for ZnPcS3 and PAlPc photosensitization especially at high concentrations (72 and 73 % of the experiments, respectively). When the neurons were sensitized with sulphonated zinc phthalocyanines ZnPcS2 and ZnPcS4 neuron response dynamics stronger depended on PS concentration. Low concentrations of these PSs (<0.1-0.05 M) caused neuron responses I or EI with the prolonged terminal inhibition leading to irreversible firing abolition in 73 and 100 % of the experiments, respectively. On the other hand, the excitatory responses E and EIE were observed in 92 and 73 % of the experiments with the high PS concentrations (>0.05-0.1 M). In line with the early assumption5,12, firing activation followed by abrupt spike abolition was caused by PD-induced plasma membrane lesion, and gradual firing suppression was the result of the photo-injury of the Ca2+-storing organelles (ER and/or mitochondria) and the following Ca2+ release. In literature13 electron microscopic study showed the mitochondria damage and intracellular vacuolization in bladder carcinoma cells under PD effect of zinc phthalocyanine. It seems, that the weak PD effect did not significantly alter the plasma membrane. However, it slowly injured Ca2+-storing organelles, and the increasing [Ca2+]i induced gradual firing inhibition. Stronger PD impact caused also plasma membrane lesion, depolarization, and firing acceleration. Therefore, AlPcS3 and PAlPc more effectively sensitized plasma membrane while sulphonated zinc phthalocyanines - intracellular membrane systems such as mitochondria and ER.
![]()
Table 3. The percentage of main types of
neuron responses to PD effect of different photosensitizers
| Photosensitizer | Concentration, M |
Neuron response type (%) E EIE EI I IE IEI |
|||||
| AlPcS3 | 0.001-0.6 |
- |
58 |
32 |
5 |
- |
5 |
0.6-12 |
22 |
78 |
- |
- |
- |
- |
|
Total: 0.001-12 |
7 |
64 |
21 |
4 |
- |
4 |
|
| ZnPcS2 | 0.001-0.05 |
27 |
- |
33 |
40 |
- |
- |
0.1-10 |
69 |
23 |
- |
8 |
- |
- |
|
Total: 0.001-10 |
46 |
11 |
18 |
25 |
- |
- |
|
| ZnPcS3 | 0.001-0.05 |
46 |
- |
- |
27 |
18 |
9 |
0.1-10 |
72 |
- |
16 |
6 |
6 |
- |
|
Total: 0.001-10 |
62 |
- |
10 |
14 |
10 |
4 |
|
| ZnPcS4 | 0.001-0.01 |
- |
- |
100 |
- |
- |
- |
0.05-50 |
55 |
18 |
18 |
- |
9 |
- |
|
Total: 0.001-50 |
38 |
12 |
44 |
- |
6 |
- |
|
| PAlPc | 0.01-0.1 |
34 |
- |
25 |
25 |
8 |
8 |
0.5-50 |
73 |
13 |
- |
7 |
7 |
- |
|
Total: 0.01-50 |
56 |
7 |
11 |
15 |
7 |
4 |
|
![]()
Table 4. Statistical parameters
characterizing the neuron lifetime dependence on PSD concentration
| Photosensitizer | Concentration range, M |
Number of experiments |
Correlation coefficient, R |
Regression coefficients lg a (lg a') b |
Student's coefficient, tst |
Fisher's coefficient, F |
|
| AlPcS3 | 0.001-12 |
27 |
0.636 |
0.83 (0.02) |
-0.325 |
4.13 |
17.0 |
| ZnPcS2 | 0.001-10 |
24 |
0.579 |
0.44 (0.44) |
-0.355 |
3.33 |
11.1 |
| ZnPcS3 | 0.001-10 |
30 |
0.587 |
0.69 (0.58) |
-0.364 |
3.84 |
14.7 |
| ZnPcS4 | 0.001-50 |
16 |
0.771 |
0.89 (0.33) |
-0.342 |
4.54 |
20.6 |
| PAlPc | 0.01-50 |
27 |
0.847 |
1.13 (0.48) |
-0.350 |
7.96 |
63.4 |
![]()
3.2. Concentration dependencies
The comparison of the dependencies of neuron lifetime on PS concentration (Fig. 1, Table 4) showed that all studied phthalocyanines were characterized with almost the same regression coefficient b: from -0.325 to -0.364. This means that one photo-excited phthalocyanine molecule creates about 3 secondary lethal lesions in the cell. These might be lipid peroxidation chains damaging different cellular membranes. It seems that such b value is the common feature of Al and Zn phthalocyanine photosensitization. Moreover, it is possible that different PS classes may be characterized with different b. It was shown, for example, that b varied from 0.22 to 0.30 in the case of neuron photosensitization with 6 different deuteroporphyrin derivatives14. Due to the approximate equality of b for different phthalocyanines, i.e. due to almost the same slope of the lines on the Fig.1, the value lg a determines the relative PD efficiency of these photosensitizers: the most effective PS has the lowest lg a and the appropriate line lies in the left lower corner in the Fig. 1. According to the Fig. 1, PD efficiency of studied phthalocyanines is increased in the following series:
ZnPcS2 > ZnPcS3 > AlPcS3 > ZnPcS4> PAlPc
The most effective PS among the studied phthalocyanines was found to be ZnPcS2. This data is consistent with its higher hydrophobicity and ability to penetrate into different cellular membranes including the membranes of ER and mitochondria. The higher PD efficiency of ZnPcS2 associated with its higher amphiphilicity and lipophilicity was also demonstrated in other papers15,16.
![]()
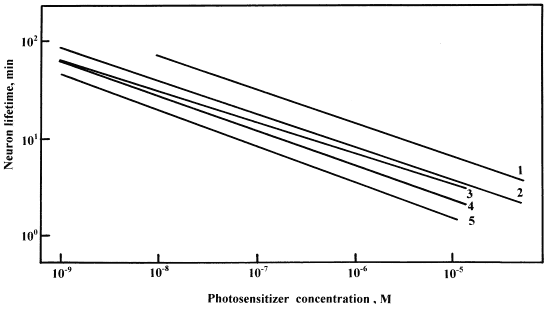
Fig.1. Neuron lifetime T (min) versus
concentrations C (M) of different photosensitizers:
(1) - PAlPc (2) - ZnPcS4; (3) - Photosens;
(4) - ZnPcS3.; (5) - ZnPcS2
![]()
The same irradiation wavelength 632.8 nm was used in all our experiments. This wavelength do not fit the absorption maximums (Lmax) of the studied PSs. However, it is of interest to compare PD efficiencies of different photosensitizers with the proviso that cells are irradiated at Lmax. It necessary to emphasize that cell death is a very complex process involving different cellular systems: genetic apparatus, proteolytic enzymes, mitochondria, plasma membrane etc. and lasting for a relatively prolonged time that varies from seconds to hours depending on the intensity of an applied impact. It is impossible to determine precisely the cell death moment and cell lifetime that can be used as a measure of cell killing efficiency. The electrophysiological criterion used in our work - the time from the irradiation onset to irreversible firing abolition which was called "a neuron lifetime" - is not a real neuron lifetime because it does not reflect biochemical and cytological processes leading to the cell death. However, the advantage of this criterion is that it can be precisely measured. We assumed that this electrophysiological "lifetime" correlates with other criteria of cell death and PD efficiency of the given PS may be characterized with the value 1/T (cell killing intensity), where T is a mean time necessary for PD-induced cell killing. This value is proportional to absorbed light intensity, Ia , and, therefore, to cell optical density: D = C e h at low cell thickness h and PS concentration C:
1/T~Ia=Io(1-e-D) ~ 0.434 Io C e h, or
-lg T = lg A2 + lg e +lg C
where Io is the intensity of the incident light, A1 and A2=0.434 Io h A1 - constants. Using k =emax/e633 (emax and e633 are PS extinction at Lmax and 633 nm, respectively), one can see that:
lg Tmax (C) = lgT633 (C) - lg k = lg a' - b lgC
and the line lg Tmax (C) lies lower than the line lg T633 (C) by lg k that is equal to the lowering of lg a by this value: lg a' = lg a - lg k . According to the numerical values of lg a' shown in the table 4 in brackets one can see that in the case of irradiation with Lmax PD efficiencies of the studied phthalocyanines can be ranged in the following series (minimal lg a' corresponds to minimal neuron lifetime, and, therefore, to maximal PD efficiency):
AlPcS3 > ZnPcS4> ZnPcS2 PAlPc > ZnPcS3
Therefore, Photosens is the most effective photosensitizer among the studied
phthalocyanines if the irradiation at Lmax is used. ZnPcS2 is
not the most effective PS because of the lack of a prominent long wavelength maximum.
![]()
4. ACKNOWLEDGEMENT
The work was partly supported by Competition Center for Fundamental Sciences at Sankt-Petersburg University.
![]()
5.REFERENCES
1. E.Ben-Hur and I. Rosenthal, "The phthalocyanines: a new class of mammalian cell photosensitizers with a potential for cancer phototherapy", Int. J. Radiat.Biol. 47, 145-147 (1985).
2. Moan, K.Berg, H.B. Steen, T.Warloe, and K. Madslien, "Fluorescence and photodynamic effects of phthalocyanines and porphyrines in cells", Photodynamic therapy : basic principle and clinical application, T.J. Dougherty and B.W. Henderson, eds., Marcel Dekker, New York, pp. 19-36 (1992).
3. W. Henderson and T.J. Dougherty, "How does photodynamic therapy work? Photochem.. Photobiol. 55, 145-57 (1992).
4. A.B. Uzdensky, "Bioelectric changes in single neuron under photodynamic effect: comparison of different photosensitizers", IEEE JSTQE. 2, 984-988 (1996).
5. A. B. Uzdensky, "Photodynamic nerve cell killing: dynamics of electrophysiological responses and photosensitizers comparison" Proc. SPIE, 3191, 130-139 (1997).
6. E. E. Giacobini, "Chemical Studies of Individual Neurons. Neurosciences Research. II. Invertebrate nerve cel"l. New York : Acad. Press., pp. 111-202 (1969).
7. G.N. Akoev and N. P. Alekseev, "Functional organization of mechanoreceptors", Nauka, Leningrad, 1985. (in Russian).Uzdensky A.B. Laser microirradiation of single nerve cell // Proc. SPIE. 1993. V.1882. P.254-267.
8. A.B. Uzdensky, "Laser microirradiation of single nerve cell", Proc. SPIE, 1882, 254-267 (1993).
9. C.A.G Wiersma, E. Furshpan, and E. Florey, "Physiological and pharmacological observations on muscle organ of the crayfish, Cambarus clarkii Girard", J. Exp. Biol. 30, 136-151 (1953).
10. G.A. Meerovich, E.A.Luk'yanets, O.A. Yuzhakova, O.L. Kaliya, G.N. Vorozhtsov, V.B. Loschenov, N.L. Torshina, A.A. Stratonnikov, E.A. Kogan, "Photosensitizer for PDT based on phosphonate phthalocyanine derivative," Proc. SPIE, 2924, 86-90 (1996).
11.B.M.Vladimirsky, Mathematical Methods in biology, Rostov Univ. Press, Rostov-on-Don, 1983. (in Russian)
12. A.B. Uzdensky, A.A. Zhavoronkova and O.Y. Dergacheva, "The photodynamic effect of Photosens on single nerve cell: chemical modification", 7th Biennial congress of International Photodynamic Association" 7-9 July 1998. Nantes. France. CD-ROM Proceeding. P45 (1998).
13. R.Bachor, E.Reich. P.Graf, A.Ruck, and R.Hautmann, "Comparison of two phthalocyanines for PDT", Proc. SPIE 2625 (1996) 395-403.
14. A.B.Uzdensky, A.V.Ivanov, A.V.Reshetnikov, G.V.Ponomarev, O.Yu.Dergacheva, A.A.Zhavoronkova, "Photodynamic effect of deuteroporphyrin IX derivatives on isolated nerve cell", Proc. SPIE, (1999) (in press).
15. P. Margaron, M.J.Gregorie, V. Scasnar, H. Ali, and J.E. van Lier, "Structure-Photodynamic activity relationships of a series of 4-substituted zinc phthalocyanines", Photochem. Photobiol. 63 (1996) 217-223.
16. M. Ochsner, "Photophysical and photobiological processes in the photodynamic therapy of tumours", J. Photochem.Photobiol. B. Biol. 39, 1-18 (1997).