| Papers and Posters | Site Home Page |
PHOTOINDUCED CROSSLINKING PROCESS OF PVC FILMS, DOPED WITH SOME 2- PHENYLBENZOTIAZOLES
N. SERTOVA, I. PETKOV*, M. EVSTATIEV, T. DELIGEORGIEV
University of Sofia, Department of Organic Chermistry, 1 James Bourchier Avenue, 1126, Sofia, Bulgaria
*
(ipetkov@chem.uni-sofia.bg: kolev_iv@hotmail.com)ABSTRACT
PVC films containing 2-phenylbenzothiazoles (BT) were UV irradiated for 3 h at normal laboratory conditions. On the basis of the results from the spectral and mechanical properties of the irradiated films and their insolubility can be make the conclusion that they undergo crosslinking upon UV-irradiation. The mechanism of crosslinking proceed in two distinct stages: interaction between HCl (product of the photodegradation of PVC) and BT – process of protonation and interaction between polyenes units of the polymer chain and dopants. In this process the dopants seem to play role of bridges between polymer chains.
Key words: PVC, polymer film, photocrosslinking, photodegradation, protonation
* The corresponding author
INTRODUCTION
The photochemistry of dyes dispersed in polymer films have been studied extensively from both fundamental and practical points of view [1-3]. Due to its widespread use and photochemical and thermal instability, poly(vinyl chloride)(PVC) has been the subject of considerable researches. However, the analysis of the literature shows that the relationship between photodegradation of PVC and the influence of the ingradients as photostabilizer or activators for photocrosslinking of PVC is unclean. The question is: at what stage the ingradient react as stabilizer and when it participates in process of the photocrosslinking. The mechanism of the photochemical degradation of PVC is, in itself, a complex problem. In many cases the photophysical and photochemical properties of these stabilizers are little known and their mode of action is obscure.
In this work, we addressed two major challenges, namely: a/ BT as consumer of HCl - photostabilizer and b/ the participation of these compounds in process of photocrosslinking. Little works has been done on the relationship between the photostabilization of the polymers and the typical chemical process of the crosslinking. Owing to their high molecular weight, polymers undergo some chemical modifications that may produce important modifications of their physical properties. Moreover, polymers contain various amounts of photocrosslinking parts that give them some physical-chemical or technological particularities. These studies on the connection between the two functions of the ingradients in the polymers are of interest, not only for the manufacture of plastic materials, but for the consumer as well.
We wish to report here the study on the photocrosslinking of PVC induced by 2-phenylbenzothiazols. The aim of this work has been to study the behaviour of the 2-phenylbenzothiazoles as photostabilizers or activators of the photocrosslinking of the PVC. The purpose of our work is to give light on the influence of the some organic compounds incorporated in PVC on the their double function.
EXPERIMENTAL
PVC films containing 2-phenylbenzothiazoles were cast from (CH2)2Cl2 solution on glass plates. The films with thickness d = 30-40m m were irradiated with unfiltered UV light (medium-pressure mercury source). Both control and irradiated PVC samples were dissolved in CHCl3, filtered and dried to constant weight.
Mechanical test was carried out at room temperature, at a strain rate of 5 mm/min using a Zwick 1464 tensile tester. The Young`s modulus E, tensile strength s and ultimate strain e were determined from the load-defformation curves. All values are averaged from five measurements.
RESULTS AND DISCUSSION
The PVC is an amorphous polymer; nevertheless its stiffness at ambient temperature is due to the attraction between electronegative chloride atoms of the macromolecular chain and electropositive hydrogen atoms of the neighboring chains. So, it is obvious that both the amorphous character and the presence of a relative amount of additives in PVC will lead to a softening of the PVC instead of a fusion upon increase of temperature. We concluded that, in the present study, the thermal behaviour could not be the only criterion to be taken into account for a comparison between irradiated and nonirradiated PVC. Therefore, the PVC photochemical behaviour study was carried out by spectral and mechanical properties to obtain new information on the photochemical crosslinking of this material. During the analysis of the results we observed a release of hydrogen chloride and the material turned deep colour as the reaction proceeded.
All these observations have suggested that three processes are likely to occur after irradiation:
1. Dehydrochalogenation and formation of double bonds in polymer sequences[4] (Scheme 1).
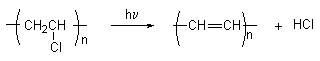
Scheme 1
The principal pathway for the non-oxidative thermal or photochemical degradation of poly(vinyl chloride)(PVC) is a dehydrochlorination process which results in the introduction into the polymer of a distribution of unsaturated polyene sequences of various lengths. As the values of n reach about 5-6 , absorption of light by the sequences in the visible region (l ³ 400 nm), becomes significant even for small (~ 0.1%) extents of degradation and the polymer begins to appear coloured. In oxidative environments additional bond scission and crosslinking reactions occur which involve the intermediacy of peroxy and hydroperoxy macroradicals and lead to undesirable changes in the polymer’s physical and mechanical properties. In spite of intensive investigations and although considerable progress has been made, the mechanisms by which the processes are initiated are not completely understood and a number of possibilities have been proposed[5]. One of these, an ionic mechanism which involves the participation of polyenylic cations, owes its origin to the known catalytic effect of strong electrophilic reagents such as AlCl3 on the rate of thermal degradation[6]. A formal similarity exists between degraded PVC containing polyene sequences, which may act as sites for additive organic compounds make them the subject of intense current interest.
2. Interaction between HCl and BT -protonation of the compounds into the polymer film - in ground or excited state(Scheme 2).
![]()
Scheme 2
3. Crosslinking with the participation of BT.
UV light is energetic factor and it can give development of other processes. It is too interesting to know whether during the irradiation time presence different than the protonation processes. This information is very important for the practical application of the results to see the gradients keep their own individual structure or the structure change drasticaly. In our case the compounds are consumers of HCl and act as photostabilizer (react with HCl, which act as autocatalyst of the process of degradation). In this connection one of very important process here is the photocrosslinking.
The spectral behaviour of the irradiated PVC films shows effective interaction between HCl and BT. It is clear that the double bonds play active role in the process of crosslinking. The evidence of that conclusion is fact that in the UV spectra of the irradiated films there are not maxima for coniugated units. PVC films contents some 2-phenylbenzothiazoles (0.125-1wt%.) (BT) are irradiated with polychromatic UV light during 3 hours (Scheme 3).
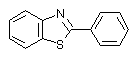
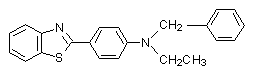
Compound 1 Compound 2
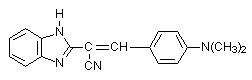
Compound 3
Scheme 3
The compounds are selected with one, two and three nitrogen atoms in the structure. The selection is not accidentally. Since the first process in PVC is the protonation, the presence of more than one basic centre (consumer of HCl) will permit to be show up the other processes.
In Table 1 are presented data for the contents of BT in irradiated and unirradiated films.
Table 1
Table 1. Contents of BT in irradiated and unirradiated polymer
films after extraction with Soxlet.
Compounds |
Residual quantity in polymer |
compound in % |
unirradiation |
irradiation |
|
1 |
52 |
33 |
2 |
31 |
67 |
3 |
17 |
62 |
The irradiated and unirradiated films extract with dichloroethane at the same conditions. Since the compounds have not chemical bond with the polymer chain, in fact in the solution can be found the quantity substance, which can not be detained in polymer. The detention can be realized through accumulation in the separate parts(amorphous parts) or through some weak interactions with separate fragments of the polymer chain. The sediment dry to constant weight. The difference in the quantity of the non extracted and extracted, irradiated and unirradiated polymer films in percentages is the substance detained in polymer. The molecules with smaller size of the structure will occupy the small volumes in the polymer mass and the extraction can not extract them. The detention of the large molecules will be difficult, as it can see for compound 2 and 3. In unirradiated films that is the main factor, which influence on the detention of the compounds. The polarization of C ® Cl bond is the reason the interactions as:(Scheme 4)
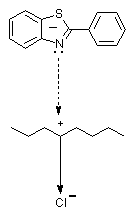
Scheme 4
to be effective. That will detain additionally the small molecules into polymer. Evidently, the large molecules will realized such interaction difficult, since they can not reach to these parts of the polymer chain. In irradiated films situation is changed strongly. The increase of the quantity of the substance, which is detention into the polymer during the irradiation from the smaller (compound 1) to more large molecules (compounds 2 and 3) show that there is seriously change in the structure of the polymer. UV irradiation is reason the compounds to be detained in polymer. If it is corectly that the light destroy the polymer(photofragmentation) than the detention will be uneffective. The low molecular compounds and parts of the polymer chain will be extracted.
The effect of UV irradiation on mechanical properties was measured by determining breaking angles according to ASTM method. The data in Table 2 demonstrated significant stabilizing ability after the UV irradiation.
Table 2
Table 2. Data for the mechanical properties of the irradiated and
unirradiated PVC films, doped with BT.(0.25 wt.%, 3 h. irr.) on the base of ASTM method.
Parameter |
Comp. 1 |
Comp. 2 |
||
unirr film |
irr film |
unirr film |
irr film |
|
s , N/mm2 |
67.3 |
56.5 |
54.4 |
63.9 |
e % |
3.6 |
9.7 |
16.7 |
11.5 |
E, N/mm2 |
1351 |
1237 |
1276.5 |
1438.3 |
where s is tensile strength, e - ultimate strain, E = s /e young's modulus.
The comparing of the data for the model compounds permit to be make the follow conclusions. The increase of the values of s during the irradiation is connected with the process of crosslinking or crystallization. The reverse is evidence for destruction. At the same time, as much polymer is more crosslinking, much the ultimate stain is lower. With other words, the increase of e during the irradiation lead to the increase of the amorphous mass and the destruction is more effective. In the end, the higher values of E show that the strength increases, the elasticity decreases and that can be written of the presence of process of crosslinking.
As that can be seen compound 2 is responsible for the crosslinking of PVC. The compound 1 activate the destruction of polymer. That conclusion is in accordance with the results of Petkov[7] for the role of some unsubstituted 2-phenylbenzothiazoles (with one basic centre) as activators of the photoinduced dehydrohalogenation of PVC. The presence of second basic centre change the situation. For example, for the compounds with structure:(Scheme 5)
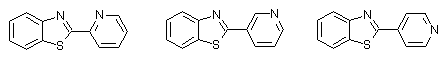
Scheme 5
the process of photodehydrohalogenation is reduced in comparison with the process of crosslinking[8]. In this process BT play the role of the catalyst.
On the base of the spectral and mechanical properties of the irradiated films can be make the conclusion that in PVC take place process of crosslinking in the presence of BT with more than one basic centre. The detention of the compounds can be on the base of the inclusion of BT in the crosslinking structures. The proof of that is the decreased of the solubility of the irradiation polymers and the specral analysis of the filtrates, which show decrease of the contents of BT in comparison with the unirradiated films. There are two possibilities for the explanation of the detenation. The first is connected with formation of free volumes into polymer where the compound can be detained(Scheme 6).
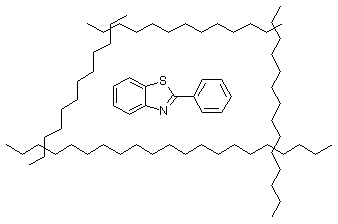
Scheme 6
The second possibility suppose chemical reaction between BT and destroyed polymer chain. The bigger percentage of the quantity substance detained in polymer after irradiation for the compounds with more large molecule(compounds 2 and 3) is seriously proof that they participate active in the crosslinking of the polymer. BT can realize the chemical bond with the polymer chain and can play role of bridges between the chains(Scheme 7):
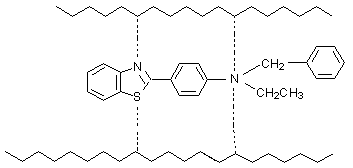
Scheme 7
The spatial crosslinking of the polymers is the reason of the decrease solubility, bigger hardness and superficial strenght.
Our results and conclusions for the role of BT in the process of crosslinking are in according to the results of M. Sindler[8] for the UV initiated interaction of some benzothiazoles with alkenes or alkines (Scheme 8):
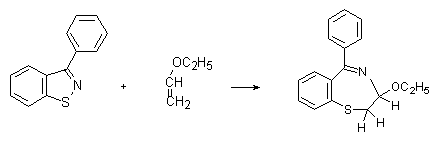
Scheme 8
In our case polyene fragments in the irradiated PVC will play the role of the alkene in the reaction(Scheme 9).
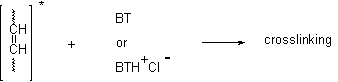
Scheme 9
The spectral behaviour of the irradiated PVC films show effective interaction between HCl and BT. This process of protonation is very effective during the first 30-40 minutes. In fact after this period evaluation of HCl continue and it can be found inside the polymer film. The appearance of colour of the irradiated films is evidence for the interaction. It is clear that the double bonds play active role in the process of crosslinking. In UV spectra of the irradiated films there is not absorption for conujgate units. One possible explanation is that the process of photodehydrochalogenation and photocrosslinking run in parallel.
REFERENCES
1. J. L. R. Williams, R. C. Daly, Prog. Polym. Sci., 5, 61 (1977).
2. N. S. Allen, J. F. McKellar, Eds.
"Photochemistry of Dyed and Pigmented Polymers", Photochemistry of Dyed and Pigmented Polymers", Applied Science Publishers: London, 1980.3. G. Smets, Adv. Polym. Sci., 50, 17 (1983).
4. J. Allan, G. Baillie, D. Gerard and J. Birnie, Vib. Spectrosc., 1(1), 97 (1990).
5. E. D. Owen, in Developments in polymer photochemistry - 3, N. S. Allen, (ed.). Applied Science Publishers Ltd, London, 1982, p. 165.
6. Z. Mayer, J. Macromol. Sci., Revs. Maacromol. Chem., C10 (2) 263 (1964).
7. I. Petkov, T. Deligeorgiev, P. Markov, M. Evstatiev and S. Fakirov. Polymer Degradation and Stability, 33 N 1, 53 (1991).
8. M. Sindler-Kulyk, D. C. Neckers, Tetrahedron Lett., 2081 (1981).