| Papers and Posters | Site Home Page |
Enzymatic activity of metmyoglobin on the thermal back cis-trans isomerization of stilbazolium betaine M.
A.V.Pastukhov
Institute of Problems of Chemical Physics RAS, Chernogolovka, Moscow region, 142432 Russia
e-mail: pastukh@cat.icp.ac.ru
Design of photochrome-protein complexes possessing photoregulated properties is a promising and intensively investigated area of contemporary photochemistry and photobiology. On the other hand, dark thermal reactions within these complexes have been less studied. This communication presents the results of a study of the “enzymatic” effect of association of a merocyanine dye, stilbazolium betaine M (SBM), with metmyoglobin (metMb) on the back thermal cis ® trans isomerization of the photochrome [1,2].
It is known [3], that SBM can occur in two different forms, q-type and b-type (scheme1),
Scheme 1.
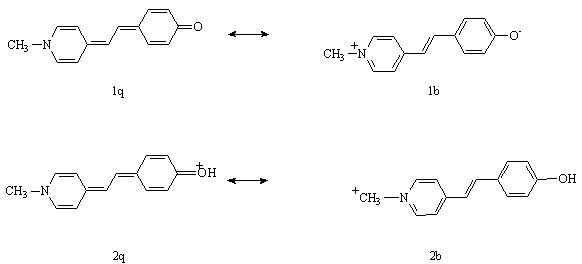
depending on polarity of a medium. The b-type structure provides a possibility of the existence of the cis- and trans-isomers. Irradiation of a solution of the pure trans-form of SBM with UV light produces a photostationary cis/trans state. Under neutral or basic conditions a spontaneous back thermal cis ® trans isomerization of SBM is then observed. Addition of metMb to the cis/trans solution accelerates significantly the back isomerization rate.
A study of the thermal isomerization rate dependence on pH and ionic strength of a solution, as well on the presence of different proteins and heme ligands was performed.
Increasing pH in the range 5.8-8.0 leads to the acceleration of the isomerization rate. An analogous effect is observed when ionic strength of a solution is changed from 1.0 to 0.02 M. Further decreasing of ionic strength somewhat lowers the isomerization rate.
Use of different heme ligands shows the gradual decrease in the thermal cis ® trans isomerization rate as the value of the equilibrium ligand associetion constant increases in the series of H2O, HCOONa, NaF, KCNS. However, there is substantional deviation from this picture in the case of NaCN and NaN3. This is possible connected with incorrected values of the equilibrium association constants for NaCN and NaN3 as it was noted by Antonini and Brunori [4].
A comparative study of the thermal isomerization of SBM in the presence of three different proteins: metMb, apoMb, and human albumin demonstrates that apoMb accelerates also the reaction rates but to a less extent than metMb does. Thus, the low polar hydrophobic heme environment plays an important role in the reaction acceleration. At the same time, the existance of similar hydrophobic binding sites in human albumin does not result in the same effect. This observation reveals a specific influence of the heme pocket on the isomerization process which can be attributed to the unique set of amino acid residues forming the heme environment.
Two possible mechanisms of the “enzymatic” activity of metMb on the thermal cis ® trans isomerization of SBM are suggested [2].
The performed investigation allows one to make the next conclusions:
1) the acceleration of the thermal cis ® trans isomerization of SBM in the presence of metMb is due to coordination of the photochrome at the sixth ligand position of Fe3+;
2) a protein globule participates in the “catalysis”of the SBM’s thermal isomerization affecning stabilization of photochrome coordination. This may be achieved not only by a sterical hindrance created by protein matrix for diffision of SBM within the heme pocket, but also by specific interactions of SBM with some amino acid residues forming the heme environment.
1. V.R.Vogel, A.V.Pastukhov, A.I.Kotelnikov. J.Fluorescence, 1999, v.9, p.209.
2. A.V.Pastukhov, A.I.Kotelnikov, V.R.Vogel. J.Photochem.Photobiol.A, (submitted).
3. U.Steiner, M.H.Abdel-Kader, P.Fischer, H.E.A.Kramer. JACS, 1978, v.100, p.3190.
4. E.Antonini, M.Brunori. Hemoglobin and myoglobin in their reactions with ligands. North-Holland Company, Amsterdam, 1971
.