| Papers and Posters | Site Home Page |

Regio-, Stereo-, and Enantioselectivity of the Paternò-Büchi Reaction on 2-Furylmethanols
Maurizio D'Auria and Rocco Racioppi
Maurizio D'Auria and Rocco Racioppi
Dipartimento di Chimica, Università della Basilicata, Via N. Sauro 85, 85100 Potenza, Italy
(e mail: dauria@unibas.it, racioppi@unibas.it)
Introduction
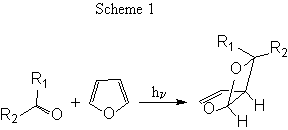
The photochemical reaction of furan with carbonyl compounds to give the corresponding oxetanes (Scheme 1) has been extensively studied in organic chemistry1 and several synthetic applications of this method have been reported.2
Some years ago Carless found that 2-acetylfurans showed a remarkable selectivity in the cycloaddition reactions with aromatic aldehydes.3
Here we want to report our results obtained using 2-furylmethanols as substrates. In literature only the reaction of 2-furylmethanol with benzaldehyde was described and, in this case, low selectivity was observed.3
Results
The irradiation of 2-furylmethanol (1a) in the presence of benzophenone (2a) gave a 8:2 mixture of regioisomeric products 3a and 4a (Scheme 2, Table 1). When the reaction was performed in the presence of benzaldehyde (2b) we observed the formation of a 2:1 mixture of the same type of regioisomeric products (3b and 4b, respectively) (Scheme 2, Table 1). This datum was in agreement with previous reported results on this compound.3
The same reaction was carried out using 2-furylethanol (1b) as substrate: in this case we observed a great increase in the regioselectivity of the reaction. In fact, when benzophenone was used as reagent only 3c was obtained as an unseparable mixture of stereoisomers (Scheme 2, Table 1). The reaction of 1b with benzaldehyde gave only the compound 3d; however, in this case we obtained the product in very low yield (Scheme 2, Table 1). Nevertheless, 3d was obtained as a single diastereoisomer. The analysis of NOE effect on this compound showed it is the 1(R,S)1’(R,S),5(R,S),6(R,S) diastereoisomer.
In order to study the effect of large side chain on the regiochemistry of the reaction, we tested the photochemical behavior of 1-(2-furyl)-heptanol (1c): in the presence of benzophenone we obtained only the compound 3e as a single couple of diastereoisomers (Scheme 2, Table 1). The study of NOE effect on this molecule showed that we obtained the 1(R,S),1’(R,S),5(R,S) diastereoisomer.
In this case we observed a high stereoselectivity in the photochemical reaction, never described before.
When the reaction was carried out in the presence of benzaldehyde, no product was observed (Table 1).
On the basis of these results we observed that both regio- and stereoselectivity enhances in the presence of hindered substituents on the side chain of 2-furylmethanols. In all the cases the most favored product was that deriving from the attack of the carbonyl group at the most hindered side of the furan ring. Finally, benzaldehyde showed a lower reactivity than benzophenone towards our substrates.
A partially different behavior was observed when we used 1-(2-furyl)benzylic alcohol (1d) as substrate. In this case, the reaction of 1d with benzophenone gave a 9:1 mixture of 3f and 4c (Scheme 2, Table 1). Also in this case we obtained 3f as a single couple of diastereoisomers. The analysis of NOE effects allows us to assign a 1(R,S),1’(R,S),5(R,S) stereochemistry to the chiral centers.
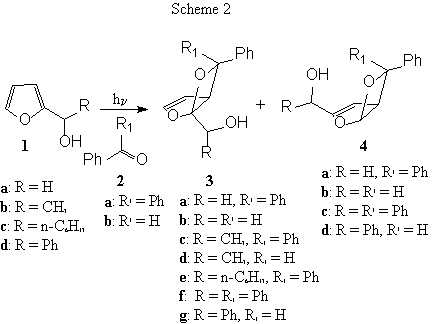
Table 1 - Photochemical reaction of 1 with carbonyl compounds
Furan |
Carbonyl compound |
Irradiation time [h] |
Product |
Yield [%] |
1a |
2a |
96 |
3a |
54 |
4a |
14 |
|||
2b |
15 |
3b |
35 |
|
4b |
18 |
|||
1b |
2a |
36 |
3c |
61 |
2b |
48 |
3d |
5 |
|
1c |
2a |
96 |
3e |
44 |
2b |
48 |
-- |
-- |
|
1d |
2a |
18 |
3f |
52 |
4c |
5 |
|||
2b |
24 |
3g |
19 |
|
4d |
31 |
When benzaldehyde was used as reagent we observed the photochemical reaction in reasonable yields (Scheme 2, Table 1). However, we obtained a 6:4 mixture of 4d and 3g: in this case, the regiochemistry of the reaction was completely reversed. The compound 4d appeared as a mixture of stereoisomers, while 3g is a 2:1 mixture of 1(R,S),1’(R,S),5(R,S),6(R,S) and 1(R,S),1’(S,R),5(R,S),6(R,S) diastereoisomers.
On the basis of the above reported data we can formulate some conclusions: 1. 2-furylmethanol derivatives react with aromatic carbonyl compounds in good yields; 2. benzaldehyde shows a lower reactivity towards 2-furylmethanols than benzophenone; 3. the reaction shows a good regioselectivity and in some cases we observed regiospecific reactions; 4. in most of the reported examples the reaction occurs on the more substituted double bond of the furan derivative; 5. the reaction is stereoselective giving, in agreement with reported data, the exo isomer when the reagent is benzaldehyde; 6. the presence of an hydroxyl group on the furan derivatives leads to different results depending on the nature of the side chain R; in particular: a. with R = CH3 the reaction does not feel the effect of the presence of the alcohol: in fact we obtained a 1:1 mixture of two diastereoisomers; b. with R = n-C6H13 we observed complete stereoselectivity: in this case, the configuration of the carbon bearing the alcohol determines the configuration of the other chiral centers on the molecule; c. with R = phenyl we observed the same behavior as in 6b with complete stereoselectivity.
Finally, we used as substrate a chiral 2-furylcarbinol. R-(+)-1d was obtained through kinetic resolution of (± )-1d in the presence of Ti(O-iPr)4, TBHP and L-(+)-DIPT (Scheme 3).
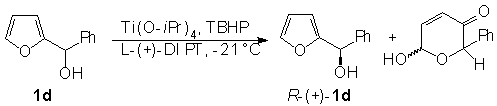 Scheme 3
Scheme 3
When the photochemical reaction was carried out on R-(+)-1d we obtained the coupling product 3f as a pure enantiomer (Scheme 4)
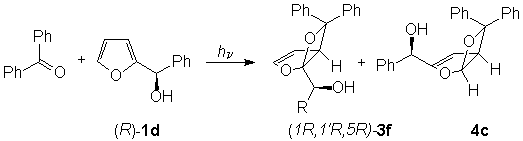 Scheme 4
Scheme 4
In Figure 1 we reported a part of a 1HNMR spectra of the compound 3f as racemate and as enantiomer, as obtained in the previous reaction.
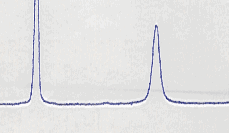
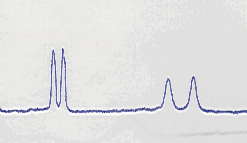
References
|
- H. A. J. Carless, F. E. Halfhide, J. Chem. Soc., Perkin Trans. 1, 1992, 1081-1082.