| Papers and Posters | Site Home Page |
ULTRAWEAK PHOTON EMISSION IN ASSESSING GROWTH FACTOR EFFICIENCY USING FIBROBLASTIC DIFFERENTIATION1
Hugo J. Niggli*a, Corinne Scalettaa, Jeff Moehlenbruckb, Fritz-Albert Popp, Yan Yuc, Renato Panizzona and Lee Ann Applegatea
aDepartment of Dermatology, Laboratory of Photobiology, University Hospital, CHUV
BT-04-423, CH-1011 Lausanne, Switzerland
bSulzer Carbomedics, 1330-B East Anderson Lane, Austin, Texas 78752 ,USA,
cInternational Institute of Biophysics, IIB, ehm. Raketenstation, Kapellener Strasse
D-41472 Neuss, Germany
1Dedicated to the memory of Alexander Gurwitsch on the occasion of his 125th birthday.
*Address for correspondence : c/o Dr. Lee Laurent-Applegate, Department of Dermatology, Laboratory of Photobiology, University Hospital, CHUV BT-04-423, CH-1011 Lausanne, Switzerland
E-mail:biofoton@mail.swissonline.ch
Running Title: Growth factor and ultraweak photon emission in human cells
Key words: Ultraweak, photon, fibroblast, skin, human, ultraviolet, growth factor
Abstract
Photons participate in many atomic and molecular interaction and changes. Recent biophysical research has shown the existence of ultraweak photons in biological tissue. It is now established that plants, animal and human cells emit a very weak radiation which can be readily detected with an appropriate photomultiplier system. Although the emission is extremely low in mammalian cells, it can be efficiently induced by ultraviolet light. Photons in the visible range are coupled with radical reactions while photons in the UV are linked with the DNA as the source. In experiments using bone growth factors (BP2, BP3 and BP 5) we tested their efficiency, in quantities as low as 5 ng, in the differentiation system of human skin fibroblast from a patient with XPA (GM05509 A) after irradiation with UVA. The different batches of growth factors showed various proliferation of skin fibroblasts in culture which could be correlated with the ultraweak photon emission. The growth factors reduced the acceleration of the fibroblast differentiation induced by mitomycin C by a factor of 10-30%. In view that fibroblasts play an essential role in skin aging and wound healing, the fibroblast differentiation system is a very useful tool in order to elucidate the efficacy of growth factors.
1. Introduction
Photons necessarily participate in all atomic and molecular interactions and changes in the physical universe. At the beginning of this century, Alexander Gurwitsch, born 125 year ago in Russia, suggested that ultraweak photons transmit information in living systems [1] as summaraized recently [2]. Although the results of Gurwitsch were refuted by Hollander and Klaus [3] and the interest for this subject declined in the following decades, the presence of biological radiation was re-examined with the development of photomultiplier tubes in the mid-fifties by Facchini and co-workers [4]. In the sixties most of the work on ultraweak photon emission was performed by Russian scientists [5-7], while in western countries several pioneers, Quickenden in Australia [8], Popp in Germany [9] and Inaba in Japan [10], independently developed methods for ultraweak photon measurements in a variety of different cells by the use of a extremely low noise, highly sensitive photon counting system which allows maximal exploitation of the potential capabilities of a photomultiplier tube. In the meantime it is commonly agreed that plant, animal and human cells emit ultraweak photons often called biophotons [11-17]. From these and additional investigations different origins for this very weak radiation have been proposed.
Most investigators think that very weak radiation results from radical reactions which can be produced by biological events such as lipid peroxidation. In studies of microsomal lipid peroxidation [18,19], it has been shown that the amount of malonaldehyde production and the intensity of emitted light are related to each other. Based on these studies, Inaba and co-workers proposed [20] that oxygen dependent light emission in rat liver nuclei was caused most likely by lipid peroxidation in the nuclear membrane. As discussed in detail by Cadenas and Sies [21], free radical decomposition of lipid hydroperoxides leads to the formation of excited chemiluminescent species by the self-reaction of secondary lipids peroxyradicals, producing either singlet molecular oxygen or excited carbonyl groups.
However, there also exists a very interesting model published in 1983 by Nagl and Popp [22] suggesting that there is a negative feedback-loop in living cells which couples together states of a coherent ultra-weak photon or biophoton field and the conformational state of the cellular DNA. The authors assume photon transfer or non-radiation chemical pumping from the cytoplasmic metabolism which results in changes of the DNA conformation via exciplex/excimer formation. Their hypothesis is based on experimental data reviewed by Birks [23] who also suggested these excimers as precursors of the pyrimidine photodimers which play a key role in the radiation damage of DNA and has been most recently proposed as biomolecular signals in communication [24]. It was hypothesized that there exists an effective intracellular mechanism of photon trapping in normal human cells [25]. This type of light-trapping mechanism in UV-induced pyrimidine dimers could be responsible for influencing metabolic and cellular events by UV-induced pyrimdine dimers once they are excised as was recently shown for photodimer induced melanogenesis [26].
Tilbury discussed in the multi-author review of van Wijk [27] that ultraweak photon emission has been detected in both the visible and ultraviolet region. Radiation in the visible region appears to be due to excited carbonyl groups and/or excited singlet oxygen dimers arising from lipid peroxidation, which in turn are associated with an increase in various reactive oxygen species such as the superoxide anion, hydrogen peroxide, hydroxyl radical and singlet oxygen. There is also substantial evidence for DNA playing a key role in these emissions as discussed above [9,28,29]. This
macromolecule may especially be involved in the emission of ultraweak photons by the cell in the UV-region of the spectrum [27].Recently, experiments with cultured human cells were reported [25] in which normal and DNA excision repair deficient Xeorderma pigmentosum (XP) cells were UV-irradiated in medium and balanced salt solution (EBSS) and assessed for ultraweak photon emission. There was some evidence of induced photon emission from normal cells in balanced salt solution, but clear evidence of a fluence-dependent emission in XP cells in medium and in EBSS were seen. Overall, it was clear that an important difference between normal and XP cells was present and it is possible that XP cells are unable to store ultraweak photons which are efficiently trapped by normal cells.
Since Hayflick's pioneering work in the early sixties, human diploid fibroblasts have become a widely accepted in vitro model system. Recently, Bayreuther and co-workers extended this experimental approach showing that fibroblasts in culture resemble, in their design, the hemopoietic stem-cell differentiation system [30]. They reported morphological and biochemical evidence for the fibroblast stem-cell differentiation system in vitro. They showed that normal human skin fibroblasts in culture spontaneously differentiate along the cell lineage of mitotic fibroblasts (MF) MFI-MFII-MFIII and post-mitotic fibroblasts (PMF) PMF IV-PMFV-PMVI-PMFVII. Additionally, they developed methods to shorten the transition period and to increase the frequency of distinct post-mitotic cell types using physical or chemical agents such as mytomycin C, 5-fluorouracil, and 5-bromo-2-deoxyuridine, that have been reported to induce differentiation in a variety of cell systems, as we have recently summarized [31-36]. Mitomycin C is an effective chemotherapeutic agent for several cancers in man; this effect is probably related to the interaction with DNA leading to DNA-DNA crosslinks and DNA-protein crosslinks. We have previously demonstrated that the mitomycin C-treatment of three different normal human fibroblast strains (CRL 1221, GM 38 and GM 1717), frequently used in mutation, transformation, and aging research, induces characteristic morphological changes in the fibroblasts and brings about specific shifts in the [35S]methionine polypeptide pattern of total cellular proteins. These results support the notion that mitomycin C accelerates the differentiation pathway from mitotic to postmitotic fibroblasts. Using this system, we were also able to demonstrate that no significant difference exists in the rate and the extent of the excision-repair response to thymine-containing pyrimidine dimers following UV-irradiation shortly after mitomycin C treatment of distinct strains of human skin fibroblasts and in the mitomycin C-induced PMF stage of these cells [31]. In addition, aphidicolin inhibits excision repair of UV-induced pyrimidine photodimers in low serum cultures of mitotic and mitomycin C-induced postmitotic fibroblasts of human skin [32]. In view that fibroblasts play an essential role in skin aging and wound healing, our results imply that the fibroblast differentiation system is a very useful tool in order to elucidate the efficiency of growth factors.
2. Material and Methods
2.1. Growth factor samples
Three different lots of bone growth factors (BP2, BP3 and BP5) in cryotubes (lyophilised; 1 mg/tube) were provided by Sulzer Carbomedics (Austin, Texas 78752, USA). In the following experiments factors BP2, BP3 and BP5 were tested at a concentration of 5 ng/ml. The mitotic cells were treated one week, while the postmitotic cells were incubated with these growth factors during two weeks. The growth factors were also added during treatment with mitomycin C to put the cells in post-mitotic status.
2.2. Cell culturing
Skin fibroblasts from a Xeroderma Pigmentosum patient of complementation group A (XPA), XP12BE (GM05509A) were obtained from the Human Genetic Mutant Cell Repository (Camden, NJ, USA). Cells were cultivated in tissue culture plastic flasks (surface, 75cm2, Gibco Basel, Switzerland) in 15 ml Dulbecco's modified Eagle's medium (DMEM; Gibco, Basel Switzerland) supplemented with 10% fetal calf serum and 100 units (U) ml-1 penicillin-streptomycin as previously described [37]. XPA cells will be plated 1:3. Passages 15-17 were used. Postmitotic fibroblasts were prepared as previously described [33]. For the determination of ultraweak photon emission in mitotic fibroblasts, 3x 105 cells were seeded into 10 cm diameter tissue culture dishes and grown for 1 week until confluency with and without growth factors at a density of 5ng/ml. UVA-irradiated fibroblasts and control cells were frozen gently in liquid nitrogen in DMEM from which phenol red has been omitted. For the determination of ultraweak photons in postmitotic fibroblasts, 1.5x105 cells were seeded into 10 cm diameter tissue culture dishes. 48 h later the medium was removed and new complete medium containing 2x10-7 M mitomycin C with and without growth factors was added for 24 h. Then the medium was removed and new complete DMEM with and without growth factors was added for 4 days. Cells were additionally treated with 2x10-7 mitomycin C essentially as described elsewhere [37]. The medium was removed and new complete DMEM with and without growth factors was added for 7 days until they have been in a postmitotic stage. Control cells which have been immediately treated with mitomycin C were also used.
2. 4. Ultraweak photon determinations
For ultraweak photon emission measurements different fibroblasts samples were transported in liquid nitrogen. Some cells were transported in T-75 flasks and used for ultraweak photon emission. The cells were thawed and were diluted in a volume of 10 ml Dulbecco's modified Eagle's medium, from which phenol red was omitted, and were transferred to a quartz sample glass (2.2x2.2x3.8 cm3; thickness 0.15 cm). Detection and registration of spontaneous and light-induced emitted photons was accomplished as described before [25]. The test sample were kept in a dark chamber in front of a single photon counting device equipped with an EMI 9558 QA photomultiplier tube (diameter of the cathode 48mm, cooled to -25° C). This high-sensitivity photon counting device is described in detail by Popp and co-workers [9,38] and measures photon intensities as low as 10-17 W in the range between 220 and 850 nm. Signal amplification is normally 106-107 and dark count ranges between 10-15 counts per second (cps). Maximum efficiency of the S20-cathode is 20-30% at 200-350nm, decaying almost linearly down to 0% at a wavelength of 870 nm and mean quantum efficiency in the entire spectral range is about 10%. The integral intensity values within each interval of 40 ms will be stored and processed by an interfaced computer. For light induced emission experiments, irradiation of the test sample will be performed perpendicular to the detecting direction with focused white light from a 150-W tungsten lamp (type 58.8297; OSRAM, Munich). Each measuring cycle was started by irradiating the sample for 5s. The measurements for the monochromatic light induction was performed by changing the spectrum of the inducing light through a monochromomator (PTI, Hamburg, Germany) from 300 to 450 nm (25nm interval).
3. Results and Discussion
- Bone factor induced proliferation in fibroblastic differentiation
Proliferation of fibroblasts was determined by counting cultured cells following a one to two week treatment with 5 ng of each lot of bone growth factor. The percentage of increase of cell proliferation was between 0 and 60% as shown in Table 1.
Table 1. Percentage of cell counts in immediately trypsinized monolayer fibroblasts with and without treatment with growth factors
Differentiation stage of cells |
control |
BP 2 |
BP 3 |
BP 5 |
Mitotic fibroblasts |
100% |
100% |
140% |
120% |
Mitotic fibroblasts (MMC) |
100% |
100% |
160% |
120% |
Postmitotic fibroblasts |
100% |
133% |
133% |
155% |
Growth Factor Efficiency: BP3>BP5>BP2
3.2. Ultraweak photons in mitotic and postmitotic human skin fibroblasts from a patient with Xeroderma pigmentosum (XPA)
We performed experiments in the fibroblast differentiation system with the human skin fibroblast from a Xeroderma pigmentosum patient (GM 05509A). We have previously found that postmitotic fibroblasts are significantly reduced in UV-induced ornithine-decarboxylase response [33]. In skin fibroblasts from a patient with Xeroderma pigmentosum (XPA) we detected a slight increase in the rate of thymine dimer induction formed after UV-irradiation of the MMC-induced postmitotic stage three weeks after MMC-treatmen [31]. For ultraweak photon emission, our experiments with cultured human cells in which normal and XPA cells were UV- irradiated we found an important difference between normal and XP cells indicating that XP cells are unable to store ultraweak photons which are efficiently trapped in normal cells [25]. For all these reasons, the the differentiation system with human cells to test the growth factors was made with skin fibroblasts from a patient with Xeroderma pigmentosum (XPA; GM05509A).
In previous experiments we have found that white light-induced photon re-emission relaxation dynamics are identical after successive irradiations of approximately 1 min intervals, and even several cycles of illumination and measurement do not quantitatively change the re-emission intensity for several hours [28]. The re-emission curves are hyperbolic as we have reported most recently with mouse melanoma cells [29]. These results confirm several previous investigations in plant and mammalian cells [11,15,16]. As interpreted by Li and Popp [38], the hyperbolic decay kinetics found in living systems after pre-illumination with white light may indicate coherent re-scattering of ultraweak photons due to collective excitation of nucleic acids within the DNA of the investigated fibroblasts.
As described above, Bayreuther and co-workers [30-36] showed biochemical and morphological evidence for the fibroblast differentiation system in vitro. They showed that normal human skin fibroblasts in culture spontaneously differentiate along the cell lineage of mitotic (MF) and postmitotic fibroblasts (PMF). Additionally, they developed methods to shorten the transition period and to increase the frequency of distinct postmitotic cell types using physical agents such as ultraviolet light (UV) and mitomycin C (MMC). To differentiate the human skin fibroblasts we have used mitomycin C. Mitotic skin fibroblasts are small in size in contrast to postmitotic cells which are enlarged by a factor of up to ten [33].
In the first experiments with human XPA skin fibroblasts it was found that measurable amounts of ultraweak photons can be achieved with cells densities as low as 2-3x104 cells /ml (data not shown). At these cell densities, control values after irradiation with a white light source for induction of ultraweak photons were determined in mitotic and postmitotic fibroblasts from fresh cells and compared to those of frozen cells. The results are shown in Table 2.
Table 2. White light induced ultraweak photon emission (cps/25) in immediately trypsinized monolayer fibroblasts (fresh) compared to fibroblasts transported in liquid nitrogen (frozen)*
Differentiation stage of cells |
Induced emission (fresh) |
Induced emission (frozen) |
Mitotic fibroblasts |
72 |
54.2 + 5.4 (n=6) |
Mitotic fibroblasts (MMC) |
74 |
40.0 + 5.0 (n=6) |
Postmitotic fibroblasts |
54 |
33.8+ 3.4 (n=6) |
*10 ml DMEM
Although there was a small increase in the induced photon emission from fresh cells, most probably due to the stress condition of the transport, it can be concluded that the frozen cells showed the same type of emission. Similiar results were obtained for monochromatic induction of the fibroblasts in the range of 300-450nm as shown for MMC- mitotic cells in Figure 1.
Figure 1. Monochromatic light (300-450 nm) induced ultraweak photon emission (UPE; cps/100) in immediately trypsinzed monolayer fibroblasts (fresh) compared to fibroblasts transported in liquid nitrogen (frozen)
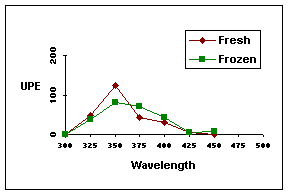
In a previous report [29], we found a slight increase in the dark count rates in postmitotic fibroblasts in XPA cells in contrast to normal fibroblasts. Therefore, the dark count rate at a cell density of 3.6x104 cells /ml in untreated and growth factor treated postmitotic fibroblasts was determined (data not shown) and this increase of the dark count rate in postmitotic cells was confirmed. From these dark count rates a decrease percentage of the acceleration of MMC-induced differentiation could be measured and it can be concluded, that the growth factors reduce the acceleration of differentiation in post-mitotic cells induced by MMC by a factor of 10-30% (data not shown).
As shown in Figure 1 the most significant increase in ultraweak photon emission after monochromatic irradiation of fibroblasts between 300-450 nm was found at 350 nm. Therefore, the induced photon emission after 350 nm light induction at a cell density of 3.6x104 cells /ml in untreated and growth factor treated MMC-mitotic fibroblasts was determined. In addition, the cells were treated with 400 kJ/m2 UVA as described elsewhere [37]. For mitotic cells, the results are shown in Table 4 showing an improvement of light-trapping ability for the growth factors in the efficiency range of BP5>BP3>BP2. For MMC-treated mitotic fibroblasts similar results have been obtained as shown in Table 5 as well as for MMC induced-postmitotic fibroblasts (data not shown).
Table 4. Induced ultraweak photon emission (%) at 350nm in mitotic fibroblasts with and without treatment with growth factors in controls and UVA-treated cells.
Differentiation stage of cells |
control |
BP 2 |
BP 3 |
BP 5 |
Mitotic fibroblasts; control |
100 |
50 |
80 |
185 |
Mitotic fibroblasts; UVA |
100 |
47 |
0 |
10 |
Growth Factor Efficiency: BP5>BP3>BP2
Table 5. Induced ultraweak photon emission (%) at 350 nm in MMC-treated mitotic fibroblasts with and without treatment with growth factors in controls and UVA-treated cells.
Differentiation stage of cells |
control |
BP 2 |
BP 3 |
BP 5 |
Mitotic fibroblasts; control |
100 |
161 |
320 |
106 |
Mitotic fibroblasts; UVA |
100 |
0 |
0 |
33 |
Growth Factor Efficiency: BP3>BP2=BP5; All very efficient
We have previously found that after white light induction there is a small decrease from mitotic to postmitotic fibroblasts from XPA-cells while there is in MMC-treated normal fibroblast a significant increase [29]. Additionally we detected that normal cells trap UV-photons [25]. As shown in Table 5, growth factor treatment indeed increases the monochromatic induction in these cells similar to normal cells. Most interestingly, UVA irradiation in mitotic and postmitotic cells significantly reduces the monochromatic induction of ultraweak photons. It can be assumed, therefore, that the decrease in mitotic untreated and MMC treated cells which has been exposed to growth factors are due to trapping of photons, emphasizing a return to normal basal cellular status.
As finally summarized, our results indicate that the tested growth factor solutions have an effect on growth and ultraweak photon emission in the differentiation model of human skin fibroblasts and provides a new and powerful non-invasive tool for the development of skin science. Its high sensitivity can be applied in all fields of skin research, in investigating skin abnormalities and in testing the effect and efficacy of regenerative, anti-aging and UV-light protective agents at very low concentrations.
Acknowledgements
This study was funded in part by grants from the Swiss National Fund (LAA and RP : 31.49120.96) and the Swiss League Against Cancer (LAA and RP : KFS 695-7-1998).
4. References
|