The introduction of axial coordinated ligands to the central atom of metal has a strong influence on p -electron conjugation of the macromolecule. In particular, axial substitution will have several effect: i) it can alter the electronic structure of the phthalocyanine; ii) it can bring in a dipole moment perpendicular to the macrocycle plane; iii) it can vary the spatial relationships between neighbouring molecules via steric effects and thus the magnitude of the intermolecular interactions. Large axial coordinated ligands are able to alter the packing of the molecules in the solid state and the tendency to aggregate in solution.
Each of these effects can influence the photo conductive and non-liner optical properties.
The aim of our current research is the synthesis of axial substituted phthalocyaninato metal complexes, where the metal is titanium (IV), zirconium (IV) and hafnium (IV), on the one hand that have sufficient solubility in water, on the other hand they potentially possess photo conductive and non-liner optical properties.
Phthalocyaninato titanium (IV), zirconium (IV) and hafnium (IV) complexes which contain gallic, 1,8-dihydroxynaphthalene-3,6-disulfonic (chromotropic), and 5-sulfosalicylic acids, as axial coordinated ligands were obtained (Figure 1).
Chemical composition of all prepared complexes is confirmed by data 1H NMR and IR spectroscopy, and results of element analysis.
This ligands were chosen so as to alter both the spatial and electronic properties of the materials, and contain groups that provide solubility in water. Compared with the chloro ligands, these substituents introduce steric crowding that give to form aggregates in water, but on the other hand the tendency to form aggregate reduces in case of transition to aprotonic solvent (DMSO). Data of UV/vis spectra confirm it (Figure 2, 3).
In conclusion stable axial coordinated, water dissolved phthalocyaninato titanium (IV), zirconium (IV) and hafnium (IV) complexes were prepared. Studies on the photo conductive and non-linear optical properties are in progress.
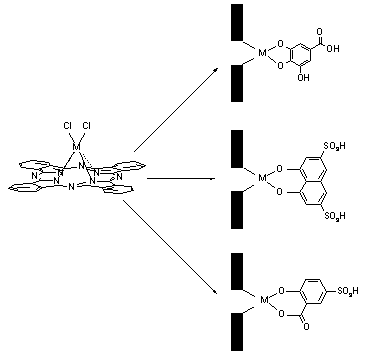
Figure 1. Synthesis of water dissolved phthalocyaninato metal complexes.
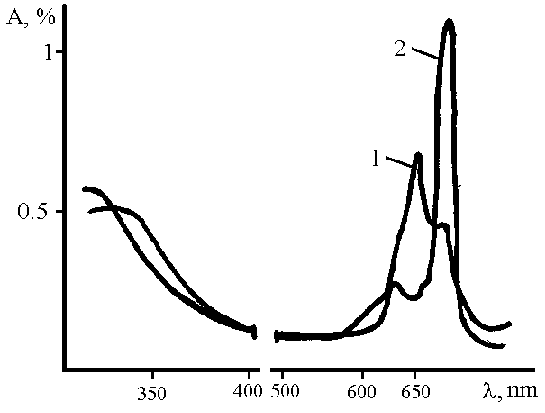
Figure 2. Typical UV/vis spectra of obtained compounds 1) in water, 2) in DMSO.
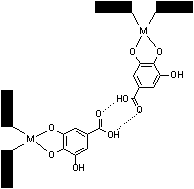
Figure 3. Suggesting structure of the formed dimer in water solution.