| Site home page | Conference home page | Discussion |
NEW TWO-FUNCTIONAL UVR SUNSCREEN PROTECTOR AND DOSIMETER
Ivan Petkov* and Jean-Michel Nunzi**
*University of Sofia, Faculty of Chemistry, Department of Organic Chemistry, 1 James Bourchier Av., 1126 Sofia Bulgaria
**DEIN-SPE, Centre d’Etude de Saclay, F-91191, Gif sur Yvette Cedex, Paris, France
New two-functional UVR sunscreen protector and dosimeter was developed on the base of PMMA film, doped with mercury-dithizone complex (HgDz2). The incorporation of the complex into a thin film of PMMA polymer forms an orange UV radiation dosimeter, which change to blue when exposed to UV radiation. The depth of the blue colour was dependent solely on the radiant exposure. The radiation-induced absorbance was measured at 490 and 600 nm as a function of UV exposure time. The dosimeter has a maximum sensitivity and suitability for use as a personal dosimeters for biologically effective solar UVB and UVA radiation. On the base of the spectral data it can well be seen that the UVB area is cover from the UV absorption of PMMA/HgDz2. The maximum sensitivity of this film lies between 270 and 315nm in the erythermal region, where most acute and chronic effects of sunlight exposure on biological systems are believed to occur. At the same time the area between 315 and 350nm is open, where the biological effectiveness of UV radiation is negligible at wavelengths above 315nm.
*Corresponding author
E-mail address: ipetkov@chem.uni-sofia.bg
INTRODUCTION
UV radiation penetrates the ozone layer over two wavelength regimes, UVB (290-320nm) and UVA (320-400nm). UVB acts directly on biological molecules, causing skin cancer, skin aging and the familiar delayed sunburn that arises 12-24 hours after exposure. In contrast, UVA acts indirectly through reactive oxygen species, causing an `immediate` sunburn that diminishes within 2 hours after exposure, and potentially plays a role in skin cancer and delayed sunburn. The biological effects of the UV radiation in humans are limited to the skin and the eye because of its low penetrating properties in human tissues. The normal responses of the skin to UV radiation may be classified as acute, e.g. erythema, melanin pigmentation and vitamin D production, or chronic, e.g. skin aging and skin cancer. Erythema (the reddening of the skin in sunburn) is a photochemical response of the skin normally resulting from overexposure to wavelengths in the UVC and UVB regions (180-315nm). Erythema induced by the longer UVB wavelengths (280-315nm) is more severe and persists for a longer period than that induced penetrating power, and so causes most of the significant adverse health effects, such as skin aging, skin cancer and eye photokeratities [1-3].
On the other side the measurement of the UV radiation is not only a periodic necessity, but also ensures that work with UV radiation can be carried out safely. Therefore, concern these effects, the development of sunscreens and sunglasses, which provide the skin and eyes with UV protection and the development of effective and simple methods and devices for the measurement of the power of the UV radiation are two very important things.
Sunscreens and sunglasses protect primarily by absorbing UV radiation, dissipating the absorbed energy as heat before it can damage photosensitive biological molecules. In addition, sunscreens and sunglasses may provide extra protection by reflecting or scattering UV radiation. The principle of the UV dosimetric systems usually is on the base of linear dependence between radiation dose and the changes in the molecules as absorption, colour, conductivity and other physical properties of the system. In many cases there is direct connection between the dose levels and the changes into the molecules. And the third very important fact is that the base of the more cases for the protection and the marked of the doses are such reactions as photoinduced processes of isomerization[4], tautomerization[5], conductivity[6], photochromism[7]. Therefore, in this connection the possibility to be combined the protector and dosimetric properties in one system is possible and can be realized excellent results.
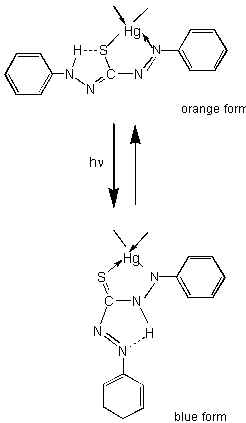
Metal dithizonate dyes are one of the earliest organic dyes that were found to exhibit photochromism. For first time Irving, et al.,[8] and Webb, et al.,[9] report independently that the mercury(II) complex is photochromic. Benzene or chloroform solutions of HgDz2 irradiated or placed in bright sunlight change the normal orange-yellow colour to an intense royal-blue colour. After stopping of the irradiation the orange-yellow colour return slowly. These colour changes can be repeated many times. After these articles the photochemistry of mercury-dithizone complex (HgDz2) and organomercury-dithizone complex (R-HgDz) have been extensively investigated [10-12]. The type of the transition is shown in Scheme 1.

Scheme 1.
The photochemical transition proceeds through a rotational movement of the dithizone in its complex with mercury.
In spite of the fact that several dyes as spiropyrans, fulgides and diarylethenes have dominated in the world of photochromism, the interest in metal dithizonate is still alive. The reasons for that are:
- possibilitity for the change of the metal atom - Mn, Os, Co, Ni, Pd, Pt, Cu, Ag, Au, Cd, Tl, Sn, Pb, Bi.
- there is a possibility, like other photochromic probes based on azo-moiety, to utilize such rotational movement of photochromic dithizone-mercury complex for characterizing the polymeric systems - molecular characterization of physical aging; analysis of free volume distribution in polymer films; analysis of segmental and side chain mobility of polymers at molecular level[13].
- that family of dyes exhibit the best fatigue resistance properties.
This paper evaluates HgDz2 in terms of colour response range on the base of its photochromic behaviour and the reversible reaction in PMMA film, in connection with the double function: dosimeter and protector under typical laboratory storage conditions.
HgDz2 was synthesized and purified by the procedure described in [10].
PMMA from POLYSCIENCES, INC was used. The polymer films doped with HgDz2 (2 wt %) were prepared on spin-coating systems. The thickness of the films was around 10 mm.
A standard OSRAM HBO 200 W/2 low pressure Hg (mercury) vapor lamp (600mW) was used for all irradiation. The whole spectrum of the lamp was used. The energy absorbed by the sample was determined by measuring the UV light intensity with a power meter in front of the probe.
The absorption spectra of the unirradiated and irradiated films were measured in the wavelength range 200-800nm using Perkin-Elmer 19 Spectrophotometer.
RESULTS AND DISCUSSION
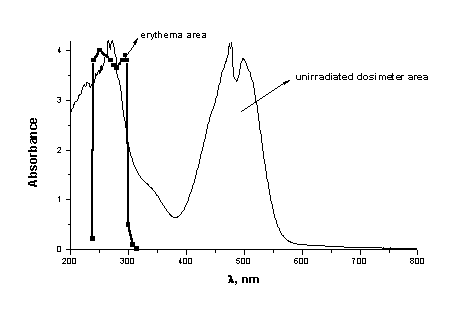
The unirradiated orange film shows an absorption band with maximum absorbance at 490nm. Absorption maximum at 600nm was not obtained (Figure 1.)
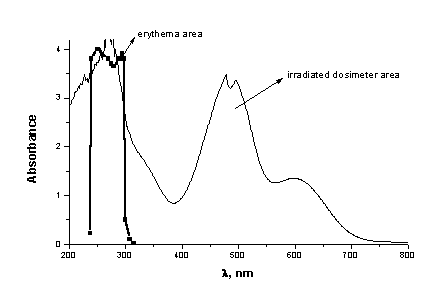
After irradiation the blue colour develops and the intensity of this colour, represented by the absorption band at 600nm, increases with increasing of the time of the irradiation (Figure 2). The changes of the absorbance agreed well with those reported in the literature [14,15].
As that was noted our idea was to use PMMA film, doped with HgDz2 as dosimeter and protector. In the course of studying the photochromism of HgDz2 as consumer of UV light we have directed our attention to two questions: the sensibility of the system and to the stability of the changes. Both these questions are very important for the development of the sensitive solar dosimeters. Only 5 seconds were necessary the photochemical transformation from orange to blue isomer reached a photostationary state (Figure 3). After exposure, the thermal back reaction of the PMMA probe was followed immediately by an UV-VIS spectrophotometer. Several hours are necessary to be reached to the initial state (Figure 4).
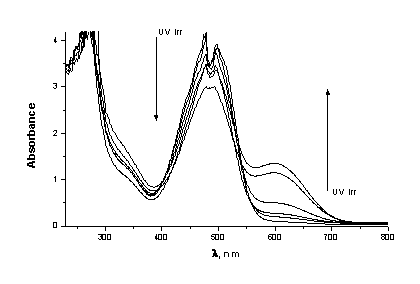
Figure 3. Variation in absorption spectra with UV light. 5 seconds UV irradiation.
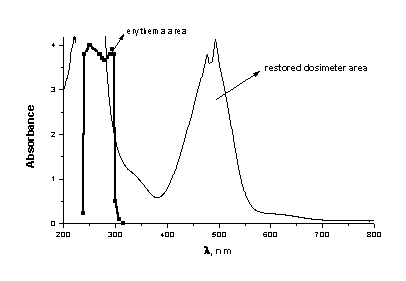
Figure 4. Restorated dosimeter area. 180 minutes after UV irradiation.
On the base of our initial spectral data it can well be seen that the UVB area is well covered from the UV absorption of PMMA/HgDz2 (Figures 1,2,3). The maximum sensitivity of this film lies between 270 and 315nm in the erythermal region, where most acute and chronic effects of sunlight exposure on biological systems are believed to occur. At the same time the area between 315 and 350nm is open, where the biological effectiveness of UV radiation is negligible at wavelengths above 315nm.
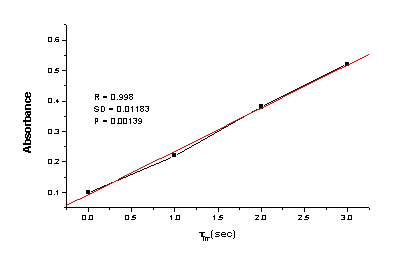
Figure 5. Dependence Abs / tirr.
The linear dependence between the absorbance and the irradiation time respectively the UV energy show that there are not side reaction and the photochromic process is the only consumer of the UV energy. It is very important to be note that alternative triplet or sensitized reactions will be relative inhibited and inefficient in the polymer film. Another essential requirement in the design of PMMA/HgDz2 system as practical UV dosimeter is that the photoproduct of the photochromic process here has a strong and distinctive visible absorption, since its visual detection is critical to dosimeter function. Performance characteristics of such system with double function will be determined in the part by the efficiency of the phototransformation of the active compound – HgDz2 in the film and the effective cover of the erythema area for protection. On the other side the spectrum of the PMMA/HdDz2 film overlaps the activation spectrum for sunburning, obtained by multiplication of the erythermal response curve of Everatt et al.[16] by the same sunlight intensity data. Within limits, it is possible to modify the activation spectrum of the protector formulation by changing the concentration of the compound, the thickness of the polymer film and the change of the metal atom in the complex. These possibilities make the investigated system exceptionally mobile. In these directions the our investigations are in progress.
REFERENCES
1. C. Baird. Environmental Chemistry; Freeman: New York, 1995.
2. H. F. Deluca, H. K. Schnoes, Ann. Rev. Biochem. 45, 631 (1976).
3. B. E. Johnson, F. Daniels Jr. and I. A. Magnus, in Photophysiology, 4, 139 (1968).
4. M. Dubois, P. Gilard, P. Tiercet, A. Deflandre and M. A. Lefebvre, J. Chem. Phys., 95, 388, 1998.
5. P. Markov and I. Petkov, Tetrahedron, 33, 1013, 1977.
6. N. Sertova, B. Geffroy, J.-M. Nunzi, I. Petkov, J. Photochem. and Photobiol. A: Chem., 113, 99,1998.
7. R. C. Bertelson, (1971) In Photochromism, (Edited by G. H. Brown), pp. 744, Wiley Interscience, New York.
8. H. Irving, G. Andrew and E. J. Risdon, J. Chem. Soc., 541(1949).
9. J. L. A. Webb, I. S. Bhatia, A. H. Corwin and A. G. Sharp, J. Am.Chem.Soc., 72, 91 (1950).
10. L. S. Meriwether, E. C. Breitner and C. L. Sloan, J. Am. Chem. Soc., 87, 4441 (1965).
11. L. S. Meriwether, E. C. Breitner and N. B. Colthuo, J. Am. Chem. Soc., 87, 4448 (1965).
12. A. T. Hutton and H. M. N. H. Irving, J. Chem. Soc. Dalton Trans., 2299 (1982).
13. B. Nayak and S. N. Gupta, J.of Pol.Sci: PartA:Polymer Chemistry, Vol. 33, 891 (1995).
14. S. S. Cooper and Sr. M. Sullivan, Anal. Chem. 23, 613 (1951).
15. G. Ivantscheff, “Das Dithizon und seine Anwendung in der Mikro-und Spurenanalyse”, Verlag Chemie, G.m.b.H., Weinheim/Bergstr., Germany, 1958.
16. M. A. Everett,R. M. Sayre, R. L. Olson, In Biological effect of Ultraviolet radiation(Edited by F. Urbach, Pergamon Press,Oxford, 181-186, 1969.