DNA photochemistry, DNA repair, and bacterial spore structure as determinants of spore resistance to solar UV radiation
Wayne L. Nicholson*, Patricia Fajardo-Cavazos, Roberto Rebeil, Paul J. Riesenman, and Tony A. Slieman**
Department of Veterinary Science and Microbiology, University of Arizona, Tucson, AZ 85721
*Corresponding author. Telephone (520) 621-2157, Fax (520) 621-6366, electronic mail address WLN@u.arizona.edu
**Present address: Department of Biology, Morningside College, Sioux City, IA 51106
SUMMARY
In the laboratory, bacterial spores are 1-2 orders of magnitude more resistant to 254-nm UV-C radiation than are vegetative cells, and UV irradiation of dormant Bacillus subtilis endospores results mainly in formation of the unique “spore photoproduct” (SP) 5-thyminyl-5,6-dihydrothymine. However, solar UV at the Earth’s surface consists of UV-B and UV-A wavelengths spanning 290-400 nm. We have been studying how the laboratory spore UV resistance model relates to spore survival to solar UV in the environment. In contrast to the laboratory model:
(i) DNA photoproduct(s) distinct from SP are formed in spores exposed to solar UV-B and UV-A radiation. These consist of double-strand (ds) breaks, single-strand (ss) breaks, cyclobutane pyrimidine dimers (Py<>Py), but not apurinic/apyrimidinic (AP) sites.
(ii) Spore DNA damage correction during spore germination is dependent upon nucleotide excision repair, recombination repair, and the SP-specific SP lyase repair systems.
(iii) The spore coat layers, particularly the inner coat, plays a protective role in the solar UV resistance of spores.
(iv) In the spore core, high levels of pyridine-2,6-dicarboxylic acid (dipicolinic acid, or DPA) enhance spore survival to solar UV.
Taken together, the data support a model in which spore resistance to solar UV results from a complex interplay of structural, photochemical, and DNA repair processes.
INTRODUCTION
The bacterial endospore is a highly-evolved structure capable of maintaining the bacterial genome in a protected, viable state for extended periods (reviewed in Nicholson et al., 2000). Spores of the common soil bacterium Bacillus subtilis have proven to be a particularly fruitful system for field studies of the consequences of long-term cellular exposure to solar radiation, due to:
1. their well-developed genetics and molecular biology,
2. their simplicity and ease of use and transport to and from monitoring sites,
3. their stability to long-term storage, both before and after exposure, and
4. the reproducibility of their inactivation response
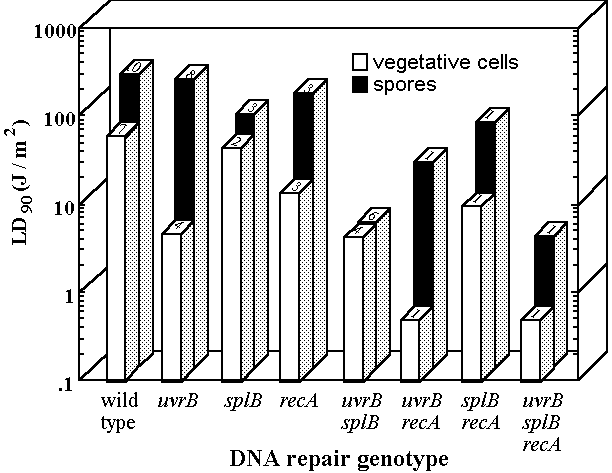
(Munakata, 1981; Tyrrell, 1978; Wang, 1991; Xue and Nicholson, 1996; reviewed in Nicholson and Fajardo-Cavazos, 1997; Nicholson et al., 2000). The nucleotide excision repair (NER), recombinational repair (Rec), and spore photoproduct (SP) lyase DNA repair pathways are major determinants of spore resistance to 254-nm UV-C radiation in the laboratory (Fig. 1).
The NER and SP lyase systems are also important for spore resistance to solar radiation, as mutant B. subtilis spores lacking both repair systems exhibit extreme sensitivity not only to laboratory UV-C radiation (Munakata, 1969), but also to the UV wavelengths present in sunlight (Munakata 1981; 1989; 1993; Quintern et al., 1994; Tyrrell, 1978; Wang, 1991; Xue and Nicholson, 1996).
|
|
Fig. 1. UV resistance of spores and vegetative cells of B. subtilis DNA repair mutants. All strains were derivatives of the prototype laboratory strain, 168. The average UV dose required to kill 90% of the population (LD90 value) is given for vegetative cells (open bars) and spores (black bars). LD90 values are averages of values reported in the literature; the number of reports from which each value was derived is listed on the top of the appropriate bar. Data are from references cited in Nicholson et al., 2000.
How well does the current laboratory model describe spore UV resistance in the environment? Solar radiation reaching the Earth’s surface is considerably more complex than artificially-produced monochromatic 254-nm UV,consisting of a mixture of UV, visible, and infrared radiation, the UV portion spanning approximately 290-400 nm (the so-called UV-B and UV-A portions of the UV spectrum) (Urbach
and Gange, 1986) (Fig. 2).
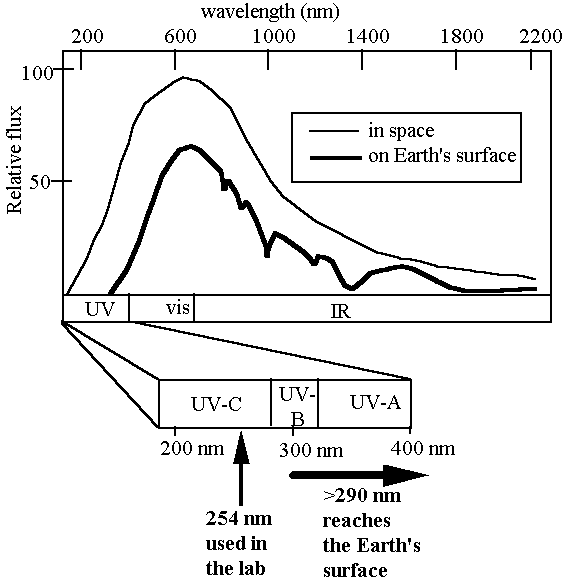
Fig. 2. Solar spectrum in space (thin line) and at Earth’s surfac (thick line).
In agreement with the current laboratory model, it has been well- documented that DNA in spores exposed either directly to solar radiation, or in the laboratory to UV wavelengths present in sunlight, accumulate mainly “spore photoproduct” (SP; the thymine dimer 5-thyminyl-5,6-dihydrothymine) as the major UV photoproduct (Tyrrell, 1978) (Fig. 3).
|
|
Fig. 3. Structures of adjacent thymines in DNA (left), cyclobutane thymine dimer (center) and spore photoproduct (rught); dRib=2’-deoxyribose.
In contrast to the laboratory model, however, in spores exposed to UV-C, UV-B, or full-spectrum sunlight, a shift towards NER is observed in the relative contributions of NER and SP lyase to spore UV resistance (Xue and Nicholson, 1996). These results were interpreted to indicate that environmentally-relevant UV wavelengths also induced non-SP photoproduct(s) in spore DNA which were preferentially repaired by NER (Xue and Nicholson, 1996). In addition, exposure of spores of NER- or SP lyase-deficient mutant B. subtilis strains to UV-A sunlight consisting of wavelengths >320 nm resulted in lethal damage which was in large part repaired neither by NER nor by SP lyase (Xue and Nicholson, 1996). Collectively, the data indicated that exposure of spores to solar radiation may produce DNA photoproduct(s) in addition to SP. We have been studying the mechanisms of spore inactivation and resistance to solar UV radiation, and have found that spore UV resistance in the environment is due to a complex set of interactions among:
(i) The photochemistry of DNA in the dormant bacterial spore,
(ii) DNA repair mechanisms which operate during spore germination, and
(iii) Protection of spore DNA damage from solar UV by spore structural components.
MATERIALS AND METHODS
Bacterial strains and culture conditions. All B. subtilis strains are derived from the author’s (W.L.N.) culture collection and have been described previously. Spores were routinely prepared by growth and sporulation for 48-72 hr. at 37˚C in nutrient broth sporulation medium (NSM)(Schaeffer et al., 1965). Suspensions of sporulated cultures were treated with lysozyme (10mg/ml) to remove vegetative cells and further purified by repeated washing in buffers and centrifugation, followed by heat shock (80˚C for 10 min) as described in detail previously (Nicholson and Setlow, 1990). The resulting spores were ascertained by phase-contrast microscopy to be > 99.9% pure.
Solar exposure. Suspensions of purified were layered either as spots on sterile microscope slides (Xue and Nicholson, 1996) or onto the bottom halves of sterile 10-cm diameter polystyrene Petri dishes (Slieman and Nicholson, 2000) and air-dried at 55˚C. The resulting dried spore samples were subjected to sunlight during the daily period of maximal solar intensity; local noon was calculated for the longitude of Tucson, AZ (111˚ 2’ W) using the Voyager II computer program (Carina Software, San Leandro, CA). For exposure to full-spectrum sunlight, samples were covered with a single layer of Saran wrap, which transmits essentially all solar UV wavelengths (Xue and Nicholson, 1996). Exposure of spores to sunlight from which the UV-B portion of the spectrum had been removed (called here “UV-A sunlight”) was accomplished by covering the samples with a 1.25-cm (1/2 inch-) thick glass plate, previously determined to completely block transmission of UV wavelengths shorter than 325 nm (Xue and Nicholson, 1996). Solar dosimetry was performed using a UVX radiometer fitted with the appropriate UV-B and UV-A probes and readings were taken under the same shielding materials which covered the spore samples. Dose rate readings (reported in J / m2 . sec) were taken at hourly intervals and the average of two successive readings was used to estimate the total UV dose (in J / m2) received by samples during the interval. In order to obtain the desired solar UV dose (especially for samples exposed to UV-A sunlight) it was often necessary to carry out exposures over the course of several days.
During the course of solar exposures, ambient temperatures exceeding 70˚C were routinely recorded (Fig. 4).
|
|
Fig. 4. Solar dosimetry performed on two typical days, 21 July, 1999 (open symbols) and 22 July, 1999 (solid symbols). Temperature (triangles), UV-A flux (circles) and UV-B flux (squares) were recorded.
Two measures were taken in order to control for potential DNA damage caused by heat. First, spore samples shielded with a single layer of aluminum foil were exposed to solar radiation in parallel and treated identically to account for heat damage. At the end of each exposure period, samples were transported to the laboratory and stored at room temperature in the dark until the following exposure period. Spore survival to solar radiation or heat was performed essentially as described previously (Xue and Nicholson, 1996; Riesenman and Nicholson, 2000). Second, B. subtilis strains which produced heat-sensitive spores were exposed to solar UV on a cooling platform connected to a recirculating ice-water bath, which maintained the temperature of the samples < 20˚C (Slieman and Nicholson, 2000).
Determination of spore survival. One million spores in 10 ml of water were spotted in triplicate on sterile glass microscope slides and the spots air dried at 37˚C for 15 min. Spores were then exposed to different treatments of UV radiation as described in detail previously (Riesenman and Nicholson, 2000; Xue and Nicholson, 1966), except as indicated below. After UV exposure, spores were recovered from the microscope slides as follows. Briefly, 0.1 ml of 10% (wt/vol) sterile polyvinyl alcohol (molecular weight, 30,000 to 70,000; Sigma Chemical Co., St. Louis, Mo.), was applied onto the dried spore spots and air dried at 37˚C for 1.0 to 1.5 hr. The resulting polyvinyl alcohol films containing the spores were then peeled from the microscope slides with a sterile scalpel and forceps and resuspended in buffer. Spores were then serially diluted in phosphate-buffered saline (Nicholson and Setlow, 1990), plated on LB agar and colonies counted after overnight incubation at 37˚C. The survival percentage of spores (%S) was calculated by the equation:
%S = (Nt/N0) x 100
where Nt and N0 stand for the numbers of CFU of the spores at the exposure time t and time zero, respectively. Spore UV resistances are expressed as LD90 values, i.e., the lethal dose of UV (in J / m2) required to inactivate 90% of the spore population, and are reported as averages + standard deviations. Differences in LD90 values were analyzed for statistical significance by Analysis of Variance (ANOVA). Differences with P < 0.05 were considered significant.
DNA isolation, manipulation, and electrophoresis. Exposed dried spore samples were resuspended in 10 ml phosphate buffered saline (PBS; 10 mM potassium phosphate, 150 mM NaCl, pH=7.4) and spores were harvested from Petri dishes using a sterile spatula. The resulting spore suspensions were collected by centrifugation, resuspended in decoating solution (8M urea, 15mM Tris base, 1% SDS, 50mM DTT) and incubated at 60˚C for 90 min to remove the protein coat. Decoated spores were washed, centrifuged, and resuspended three times with STE buffer (10 mM Tris-HCl, pH 8, 10 mM EDTA, 150 mM NaCl) and once with lysis solution (50 mM NaCl, 100 mM EDTA). Spores were then lysed and chromosomal DNA was extracted and purified as previously described (Cutting and Vander Horn, 1990). To detect Py<>Py, DNA was first digested with phage T4 endonuclease V (Endo V)(Epicentre Technologies, Madison, WI), which cleaves the phosphodiester backbone 5’ to Py<>Py. To detect apurinic/apyrimidinic (AP) sites, DNA was first digested with Endonuclease IV (Endo IV) (Epicentre Technologies) (Ljungquist, 1976). Neutral agarose gel electrophoresis of DNA was performed by standard techniques (Sambrook et al., 1989). In order to detect ss breaks generated in DNA either directly by UV treatment or as a result of Endo V or Endo IV cleavage at Py<>Py or AP sites, DNA was denatured with 300 mN NaOH (final concentration) and electrophoresed at 4˚C through 0.8% alkaline agarose gels with buffer recirculation essentially as described previously (Sambrook et.al., 1989). Migration of DNA was determined relative to a set of molecular weight standards ranging in size from 0.5 - 12 kb (1 Kb Ladder, Life Technologies, Gaithersburg, MD).
DNA photochemistry in the dormant bacterial spore. Chromosomal DNA extracted and purified from unirradiated B. subtilis spores were separated on either 0.8% neutral- or alkaline-agarose gels beside high-molecular-weight markers (phage l digested with HindIII) demonstrated either 23-kbp ds fragments or 23-kb ss fragments, respectively (data not shown); thus, DNA extracted and purified from spores was uniformly sheared to approx. 23-kbp ds fragments, and suffered no detectable additional ss breaks during the purification procedure.
Spores of strain WN356 were exposed to full-spectrum solar radiation, UV-A solar radiation, or solar heating. During the exposure period, measurements of temperature, and UV-B and UV-A flux were recorded at hourly intervals. Chromosomal DNA isolated from exposed spores was then probed for ds breaks by neutral agarose gel electrophoresis and ss breaks by denaturation in alkali followed by alkaline-agarose electrophoresis. Pretreatment of DNA with T4 Endonuclease V or Endonuclease IV was used to detect presence of Py<>Py or AP sites, respectively. The results are shown in Fig. 5.
Spores of strain WN356 covered with Saran wrap were exposed to full-spectum solar radiation on the 4th and 5th of August, 1998. The total dosage for the full spectrum irradiated spores was determined to be 8.23 x 105 J/m2 UV-A and 3.53 x 105 J/m2 UV-B, respectively. Exposure of spores to full spectrum sunlight resulted in the formation of ss breaks and Py<>Py in chromosomal DNA of spores (Fig. 5B). Ds breaks
|
|
Fig. 4. Chromosomal DNA extracted from spores of strain WN356 exposed to UV-A sunlight on 5-16 October 1998 and full spectrum sunlight on 4-5 of August 1998 was electrophoresed through a neutral 0.8% agarose gel (A) or a 0.8% alkaline-agarose gel (B). Spores are: not exposed (lanes 1 and 2); exposed in parallel to heat only (lanes 3 and 4); exposed in parallel to UV-A sunlight only (1.1 x 10 6 J / m2, lanes 5 and 6); exposed to full spectrum sunlight (8.23 x 105 J/m2 UV-A + 3.53 x 105 J/m2 UV-B) (lanes 7 and 8). Isolated DNA was treated with Endo V before electrophoresis (lanes 2, 4, 6, and 8). M, molecular weight markers. The 12 kb and 1 kb markers are indicated with arrowheads..
were also detected (Fig. 5A), but no AP sites were present (data not shown). Despite the fact that the temperature exceeded 70˚C during
the experiments, DNA damage in spores exposed to full-spectrum sunlight or UV-A sunlight was not caused by heat, as spores exposed in parallel to the same heat regimen but shielded from solar radiation exhibited no detectable DNA damage (Figs. 5A and 5B).
On clear days from 5 to 16 October 1998, spores of strain WN356 were exposed under 1.25-cm plate glass to UV-A sunlight to a total dosage of 1.1 x 106 J/m2. DNA extracted from spores exposed to UV-A sunlight and electrophoresed through 0.8% neutral and 0.8% alkaline-agarose gels was observed to contain ds breaks (Fig. 5A) and ss breaks (Fig. 5B), but virtually no Py<>Py (Fig. 5B) or AP sites (data not shown). Again, damage to spore DNA was due to direct exposure to solar radiation and not to heat, as a parallel set of spores shielded from UV by aluminum foil but exposed to the same temperature regimen accumulated no detectable DNA damage (Fig. 5).
|
|
Fig.6. A. UV treatments to which spores have been exposed. 1, artificial UV-C (254 nm); 2, artificial UV-B (290-310 nm); 3, full-spectrum sunlight (>290 nm); 4, “UV-A” sunlight (>325 nm). B. Summary of B. subtilis spore photochemistry at different artificial and environmental UV wavelengths. UV treatments are numbered as in part (A). SP, spore photoproduct; Py<>Py, cyclobutyl pyrimidine dimers; SS, single-strand breaks; DS, double-strand breaks; AP, apurinic/apyrimidinic sites. +, damage detected; +/-, damage barely detectable; -, damage not detected. *SP data are from Tyrrell (1978).
In order to understand the photochemistry of spore DNA in the environment as compared to the artificial laboratory model, spores were irradiated with full-spectrum sunlight and UV-A sunlight. In addition to the well-characterized spore photoproduct (SP), spores were observed to accumulate ds breaks, ss breaks, and Py<>Py (Fig. 6).
This study clearly showed that B. subtilis spores accumulated Py<>Py in addition to the predominant SP when subjected to environmentally-relevant full-spectrum solar radiation. Furthermore, both full-spectrum solar UV and UV-A alone induced ds breaks and ss breaks. The results suggest that it is the UV-B component of sunlight which is responsible for Py<>Py formation in spore DNA. In contrast, no apurinic/apyrimidinic sites were detected in any of our DNA preparations as revealed by digestion with Endo IV (data not shown).
DNA repair mechanisms which operate during spore germination. We have shown previously (Xue and Nicholson, 1996) that NER and SP lyase are major determinants of spore resistance to solar UV, particularly to the UV-B portion of the solar spectrum. These observations coincide well with the above data indicating that SP and Py<>Py are major photoproducts found in spores exposed to solar UV (Fig. 6). This subject has been recently reviewed extensively (Nicholson and Fajardo-Cavazos, 1997; Nicholson et al., 2000).
Protection of spore DNA damage from solar UV by spore structural components. Two major spore components have been tested for their possible contribution to spore solar UV resistance—(i) the proteinaceous spore coat layers which surround the spore, and (ii) DPA which resides in the spore core.
(i) Spore coats. Mutant B. subtilis spores lacking the inner, outer, or both spore coat layers were tested for their resistance to UV radiation both in the lab and in the environment (Riesenman and Nicholson, 2000). Spores lacking the inner coat and spores lacking both inner and outer coats were found to be more sensitive to UV-B in the laboratory and to solar radiation (Fig. 7).
|
|
Fig. 7. Resistance of spores lacking inner, outer, or both spore coat layers to UV radiation from artificial or natural sources. Data are normalized to wild-type spores for comparison. Data are taken from Riesenman and Nicholson, 2000.
(ii) DPA. DPA (dipicolinic acid; pyridine-2,6-dicarboxylic acid) is a major component of the spore core, and confers heat resistance upon spores (Paidhungat et al., 2000). We showed that DPA also confers a high degree of resistance to spores of all UV wavelengths, particularly UV-B (Slieman and Nicholson, 2001)(Fig. 8).
|
|
Fig. 8. UV resistance of spores of isogenic mutant B. subtilis strains which either produce or lack DPA. Data are normalized to DPA-less spores, and are taken from Slieman and Nicholson, 2001.
1. The resistance of bacterial spores to solar UV is the sum of a number of protective, photochemical, and repair mechanisms.
2. The spore coats and DPA both offer significant protection to inactivation of spores by solar UV.
3. DNA in spores exposed to solar UV exhibits a complex photochemistry consisting of (at least) SP, Py<>Py, ss and ds breaks, but not AP sites.
4. The NER and SP lyase DNA repair systems are important for repair of DNA damage caused mainly by the UV-B portion of sunlight.
ACKNOWLEDGMENTS
This work was supported by grants from the National Institutes of Health (GM47461) and the Arizona Agricultural Experimental Station (USDA-HATCH-ARZT-136753-H-02-116) to W.L.N.
REFERENCES
*Cutting, SM, and P.B. Vander Horn. 1990. Genetic analysis, p. 27-74. In C.R. Harwood and S.M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
* Ljungquist, Siv. 1976. A new endonuclease from Escherichia coli acting at apurinic sites in DNA. J. Biol. Chem. 262: 2808-2814.
*Munakata, N. 1969. Genetic analysis of a mutant of Bacillus subtilis producing ultraviolet-sensitive spores. Mol. Gen. Genet. 104: 258-263.
*Munakata, N. 1981. Killing and mutagenic action of sunlight upon Bacillus subtilis spores: a dosimetric system. Mutat. Res. 82: 263-268.
*Munakata, N. 1989. Genotoxic action of sunlight upon Bacillus subtilis spores: monitoring studies at Tokyo, Japan. J. Radiat. Res. 30: 338-351.
*Munakata, N. 1993. Biologically effective dose of solar ultraviolet radiation estimated by spore dosimetry in Tokyo since 1980. Photochem. Photobiol. 58: 386-392.
*Nicholson, W.L., L. Chooback, and P. Fajardo-Cavazos. 1997. Analysis of spore photoproduct lyase operon (splAB) structure and function using targeted deletion-insertion mutations spanning the Bacillus subtilis ptsHI and splAB operons. Molec. Gen. Genet. 255: 587-594.
*Nicholson, W.L. and P. Fajardo-Cavazos. 1997. DNA repair and the ultraviolet radiation resistance of bacterial spores: from the laboratory to the environment. Recent Res. Devel. Microbiol. 1: 125-140.
*Nicholson, W.L., N. Munakata, G. Horneck, H.J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Microbiol. Rev. 64: 548-572.
*Nicholson, W.L. and P. Setlow. 1990. Sporulation, germination, and outgrowth, pp. 391-450. In, Harwood, C.R. and Cutting, S.M. (eds). Molecular biological methods for Bacillus. John Wiley and Sons, Sussex, England.
*Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182: 5505-5512.
*Quintern, L.E., M. Puskeppeleit, P. Rainer, S. Weber, S. el Nagger, U. Eschweiler, and G. Horneck. 1994. Continuous dosimetry of the biologically harmful UV radiation in Antarctica with the biofilm technique. J. Photochem. Photobiol. B: Biol. 22:59-66.
*Riesenman, P.J. and W.L. Nicholson. 2000. Role of the spore coat layers in Bacillus subtilis spore resistance to hydrogen peroxide, artificial UV-C, UV-B, and solar UV radiation. Appl. Environ. Microbiol. 66: 620-626.
*Sambrook, J., E.F. Fritsch, and T. Maniatis (ed.). 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, NY, U.S.A.
*Schaeffer, P., J. Millet, and J.-P. Aubert. 1965. Catabolic repression of bacterial sporulation. Proc. Natl. Acad. Sci. USA 54: 704-711.
*Setlow, P. 1995. Mechanisms for the prevention of damage to DNA in spores of Bacillus species. Annu. Rev. Microbiol. 49: 29-54.
*Slieman, T.A. and W.L. Nicholson. 2000. Artificial and solar UV radiation induces strand breaks and cyclobutane dimers in Bacillus subtilis spore DNA. Appl. Environ. Microbiol. 66: 199-205.
*Slieman, T.A. and W.L. Nicholson. 2001. Role of endogenous dipicolinic acid (DPA) in the survival of Bacillus subtilis spores to artificial and solar ultraviolet radiation. In preparation.
*Tyrrell, R.M. 1978. Solar dosimetry with repair deficient bacterial spores: action spectra, photoproduct measurements and a comparison with other biological systems. Photochem. Photobiol. 27: 571-579.
*Urbach, F. and R.W. Gange (ed). 1986. The biological effects of ultraviolet A radiation. Praeger Publishers, New York.
*Wang, T.-C.V. 1991. A simple convenient biological dosimeter for monitoring solar UV-B radiation. Biochem. Biophys. Res. Commun. 177:48-53.
*Xue, Y. and W.L. Nicholson. 1996. The two major spore DNA repair pathways, nucleotide excision repair and spore photoproduct lyase, are sufficient for the resistance of Bacillus subtilis spores to artificial UV-C and UV-B but not to solar radiation. Appl. Environ. Microbiol. 62: 2221-2227.