M. Idrish Miah*
Biophysics Laboratory, Department of Physics, Norwegian University of Science and Technology, Gløshaugen, N-7034 Trondheim, Norway.
(E-mail: miah@stud.ntnu.no, Fax: +4773597710, Phone: +4773887773)
Abstract. Fluorescence spectroscopy was used to study protoporphyrin IX(PpIX) metabolism level in chick embryos during the cell proliferation process. The emission spectra of albumin from non-incubated and incubated eggs were measured. The relative characteristic emission intensity of PpIX was used to determine the level of PpIX metabolism as a function of the embryonic development time. This technique might be used to estimate the tumor development time.
Keywords : Protoporphyrin IX, metabolism level, cell proliferation, fluorescence spectroscopy, chick embryo, albumin, embryonic development.
1. Introduction
Porphyrins are synthetic precursors to the prosthetic group heme in hemoglobin and protoporphyrin IX (PpIX) is an intermediate in the biosynthesis of heme and can be amassed since the conversion process into heme is slow. The factors which control the biosynthesis and accumulation of PpIX are not yet completely understood [1-2]. Much of what we know on this topic has come from studies of cancer cells, where the control of cell proliferation is disrupted. These cells are mutants, and because they proliferate excessively, they give rise to tumors [3-4]. Analysis of the genetic alternations in cancer cells has revealed a large number of genes that encode proteins involved in the control of cell proliferation [5]. Uncontrolledly abnormal growth of cancer cells indicates that the accumulation of PpIX may be due to the proliferation of cells [6-7]. Abnormal metabolism of PpIX has been observed in the blood, plasma, serum and tissue of cancerous patients, which indicates that cancer cells accumulate substantially more PpIX than the normal cells and tissues [6-10].
The disease occurs in humans and animals will affect the metabolism and the regulation of organic material inside the body [11]. By investigating the metabolic process, the disease can be diagnosed. The spectral ratio of the characteristic emission of PpIX can be used to monitor change in the metabolism since the fluorescence intensity is linearly dependent on the concentration of the luminous material [12].
The egg of a chick is the most remarkable of animal cell. Under appropriate environmental conditions, the fertilized eggs start mitosis [13]. The embryo will obtain nutrition and energy from the albumin and release the products of metabolism into it [14-15]. By analyzing the albumin, the metabolism level of PpIX could be investigated. In the present work fluorescence spectra of albumin from different developmental stages have been measured to relate metabolism level with the embryonic development time from the relative characteristic emission intensity of PpIX. The main objective of this study was to understand the PpIX metabolic process in the developing tumor and to search for a possible method for estimating the stages of the tumor development
2. Materials and Methods
Thirty two chick embryos of different sizes were divided into four groups of eight each: group 1(58-59 g), group 2(60-61 g), group 3(62-63 g) and group 4(64-65 g). To avoid mixing up, these groups were labeled. Four eggs from each group were incubated in an incubator (CO2-Incubator) at
37oC in 60% humidity and 0.5% CO2 to achieve embryonic development [14]. The rest of the non-incubated eggs were used as non-fertilized. Fertilized eggs were washed with ethanol (70%). A hole was drilled in the smaller apex and 1 ml of albumin was aspirated from a group of four eggs. The sample was placed in a quartz cell for fluorescence measurement. Emission spectra from albumin were measured using a spectrofluorometer ( Perkin-Elmer Luminescence Spectrometer, LS 50B) under the excitation at 410 nm. This procedure was repeated every two days for subsequent groups.
3. Results and Discussion
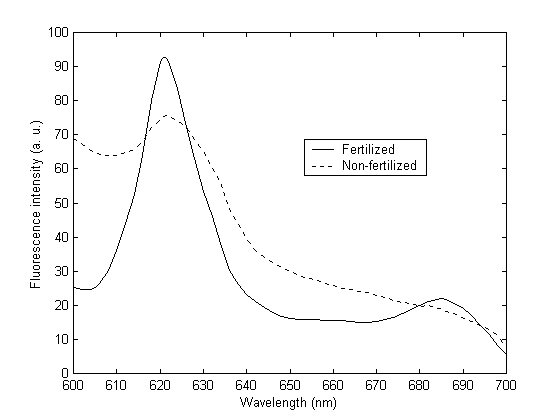
Figure 1 shows a typical fluorescence spectrum from the albumin sample. The solid line represents the emission from albumin of fertilized egg, while the dashed line represents that from non-fertilized egg. A strong
fluorescence emission at 621 nm was observed. This is the characteristic emission of PpIX [12]. It was found that the relative emission intensity increases during the cell proliferation process, indicating an increase in the concentration or in the metabolic level of PpIX. This indicates that the cell proliferation affects the PpIX metabolism [11]. The level of PpIX metabolism in fertilized eggs was studied by measuring fluorescence intensity of the albumin. The level of metabolism defined as the ratio of the relative emission intensity of PpIX was calculated from the peak fluorescence intensities from albumin of fertilized egg and non-fertilized egg, respectively. The embryonic development time with the level of metabolism is shown in Figure 2. During the initial phase of mitosis, the cell proliferation rate is very high and PpIX accumulates, the PpIX metabolism level increases linearly with time, as indicated by the fitting line (solid line in Figure 2). When organ development starts, nutrition and energy are consumed from the albumin and, as a result, the rate of cell proliferation will slow down. The PpIX metabolic level decreases with time after reaching its maximum value around 12 day. This is due to the exhaustion of nutrition and energy stored in the albumin.
In biological systems, PpIX is an intermediate in the biosynthesis of the F2+ containing heme, a prosthetic group of proteins. Heme biosynthesis is an essential pathway in cell metabolism. Due to the excessive cell proliferation and division and the slow conversion into heme from PpIX, both cancer cells and tissues will accumulate substantially more PpIX than the normal cells and tissues [6-10]. The process of PpIX metabolism in cancer cells and tissues (tumor) is very similar to the chick embryonic development and may be used to investigate the stages of the tumor development.
4. Conclusions
PpIX metabolism was studied in the cell proliferation process in chick embryos by measuring photoluminescence. Characteristic fluorescence emission of PpIX at 621 nm was used to estimate its metabolic level. It was observed that the relative emission intensity increases during the initial phase of the cell proliferation and division, indicating the PpIX accumulation occurs. The experimental data of metabolic level (for initial phase) was fitted to a linear curve, which allows us to calculate the metabolism level with time in chick embryos during the initial phase of cell proliferation. The model of cell proliferation in chick embryos may
be used to estimate the growth of cancer cells and tissues in tumor. This suggests that this technique may be useful for estimating the tumor development time and may provide an early diagnosis for cancer.
Acknowledgements
Work in our laboratory would not have been possible without such outstanding researchers as Professors T.B. Melø and A. Johnsson.
References
1. Kondo, M., Hirota, N., Tokaoka, T. and Kajiwara, M. : Heme-biosynthetic enzyme activities and porphyrin accumulation in normal liver and hepatoma cell line of rats, Cell Biol. Toxicol. 9 (1993), 95-105.
2. Gibson, S.L., Cuprisk, D.J., Havens, J.J., Nguyen, M.L. and Hilf, R. : d-Aminolaevulinic acid-induced photodynamic therapy inhibits protopophyrin IX biosynthesis and reduces subsequent treatment efficiency in vitro, Br. J. Cancer. 80 (1999), 998-1004.
3. Kennedy, J.C., Pottier, R.H. and Pross, D.C. : Photodynamic therapy with endogeneous protoporphyrin IX : basic principle and present clinical experience, J. Photochem. Photobiol. B Biol. 6 (1990), 143-148.
4. Moan, J., Berg, K., Gadmar, Ø.B., Iani, V., Ma, L. and Juzenas, P. : The temperature dependence of protoporphyrin IX production in cells and tissues, J. Photochem. Photobiol. B Biol. 70 (1999), 669-673.
5. Hartwell, L. : Defects in a cell cycle checkpoint may be responsible for the genomic instability of cancer cells, Cell. 71 (1992), 543-546.
6. Heyerdahl, H., Wang, I., Liu, D.L., Berg, R., Andersson-Engels, S., Peng, Q., Moan, J., Svanberg, S. and Svanberg, K. : Pharmacokinetic studies on 5-aminolevulinic acid-induced protoporphyrin IX
accumulation in tumors and normal tissues, Cancer Lett. 112 (1997), 225-231.
7. Malik, Z., Kostenich, G., Roitman, L., Ehrenberg, B. and Orenstein, A. : Topical application of 5-aminolevulinic acid, DMSO and EDTA : protoporphyrin IX accumulation in skin and tumors of mice, J. Photochem. Photobiol. B Biol. 28 (1995), 213-218
8. Rick, K., Sroka, R., Stepp, H., Kriegmair, M., Huber, R.M., Jacob, K. and Baumgartner, R. : Pharmacokinetics of 5-aminolevulinic acid-induced protoporphyrin IX in skin and blood, J. Photochem. Photobiol. B Biol. 40 (1997), 313-319.
9. Spikes, J.D. and Straight, R.C. : Photodynamic behavior of porphyrins in model cell, tissue and tumor systems, Libreria Progetto, Padova, 1985.
10. Doiron, D.R. and Gomer, C.J.: Porphyrin localization and treatment of tumors, Alan R. Liss., New York, 1984.
11. El-Sharabay, M.M.H., El-Wassel, A.M., Hafez, M.M. and Salim, S.A. : Porphyrin metabolism in some malignant deseases, Br. J. Cancer. 65 (1992), 409-412.
12. Corwin, A.H., Chivvis, A.B., Poor, R.W., White, D.G. and Baker, E.W. : The interpretation of porphyrin and metalloporphyrin spectra, J. Am. Chem. Soc. 90 (1968), 6577-6583.
13. Wadsworth, P. : Mitosis . spindle assembly and chromosome motion, Curr. Opin. Cell Biol. 5 (1993), 123-128.
14. Austin, C.R. : The egg. In reproduction in mammals : I. Germ cells and fertilization, Cambridge, U.K. : Cambridge University Press, 1982.
15. Garbers, D.L. : Molecular basis of fertilization, Annu. Rev. Biochem. 58 (1989), 719-742.
 |
Figure 1. Fluorescence spectra from protoporphyrin IX. Solid line and dashed
line represent the emissions from albumin of fertilized egg and non-fertilized
egg respectively. The excitation wavelength was 410 nm.
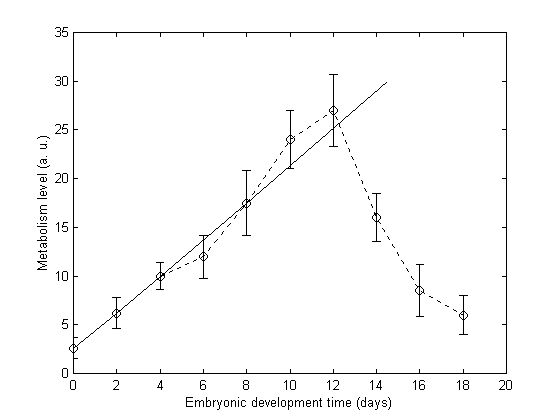 |
Figure 2. Protoporphyrin IX metabolism level as a function of the embryonic development time (dashed line). Bars show standard errors from four indep-
endent experiments (groups). Solid line is fitted to the data during the initial
phase of cell proliferation.
* Permanent Address: Department of Physics, Bangladesh Institute of Technology, Chittagong, Chittagong-4349, Bangladesh. Fax: +880 31 714910, Phone: +880 31 714947.