| Site home page | Conference home page | Discussion |
Back reflected photons and Carbon Monoxide Enhance Chemiluminescence in non-diluted human blood: evidence in favour of red and white cells interactions
V.L. Voeikov, R. Asfaramov, Yu.S. Bulargina, C.N. Novikov, N.D. Vilenskaya.
Faculty of Biology, Moscow State University, Moscow 119899, Russia. E-mail: vvl@soil.msu.ru
Abstract
We demonstrate here, that parameters of luminol- or lucigenin-dependent chemiluminescence (LM-CL and LC-CL, respectively) in whole non-diluted human blood during the respiratory burst (RB) development are influenced by photons reflected back to the sample. Back reflected photons accelerate slowly developing and retard rapidly developing RB at the stage of its development, and at the stage of RB decay they prolong the process. As demonstrated by histochemical NBT test, self-irradiation of blood influences neutrophil activity in it. We suggested that back reflected photons excite hemoglobin (Hb) molecules and that energy of excitation may be used for enhanced oxygen release from many HbO2 molecules simultaneously due to their tight package in erythrocytes. An increase in oxygen availability facilitates reactive oxygen species generation by white blood cells related to photon emission from blood. This speculation was supported in studies of the effect of carbon monoxide upon whole blood and isolated neutrophils. While CO sharply intensified LC-CL in non-diluted blood, it had much weaker effect in saline-diluted blood, and inhibited LC-CL in neutrophil suspension. CO is known to bind to heme much more tightly than O2. Whereas in neutrophil suspension it binds to heme enzymes responsible for oxygen reduction, in blood its major target is deoxy-Hb. Presumably, high energy released in heme-CO reaction in erythrocytes induces accelerated oxygen release from many HbO2 molecules resulting in the similar effect upon CL from whole blood as that produced by blood self-irradiation. These results indicate that there are complex interactions between different cellular components of blood.
Introduction
Stimulated neutrophils and other phagocyting cells react to multiple stimuli by escalation of reactive oxygen species (ROS) production followed with chemiluminescence (CL) [1]. As intensity of photon emission is very low, CL indicators, such as luminol or lucigenin are introduced into a cellular suspension to increase quantum yield of CL [2]. Lucigenin is regarded as a relatively selective probe for superoxide anion radical (O2· ¾) [3], while luminol reports of a variety of reactive oxygen species (H2O2, HClO, OH·) production [4].
Ultra-weak CL in the optical spectral range from many cells is generally thought to represent random imperfections accompanying normal physiological processes of oxygen consumption, and no biological function is currently ascribed to it. However evidence is accumulating that electron excited species are regular products of some biochemical processes and that energy of their relaxation may be used by living systems in different ways, such as provision of excitation energy for endothermic chemical reactions, photomodulation of enzyme activities, ets [5]. Energy of electron excitation may be transmitted from the sites of its generation (donors) to the sites of its utilisation (acceptors) both by radiation-less as well as by radiative mechanisms[6].
Recently we reported that under some circumstances highly significant photon emission can be registered from non-diluted human blood, and its intensity may increase enormously upon addition of luminol [7]. RB induction in blood in the presence of luminol was always followed with a great elevation of photon counts rate. Despite a very low optical transparency of non-diluted blood in the range of the emission range of luminol, maximal counts rate attained was often exceeding that observed in suspensions of neutrophils containing the same quantity of cells. Parameters of photon emission from blood depend more on its physiological state and metabolism then on its optical properties[8]. LC-CL can also be registered from non-diluted human blood even without any additives inducing RB of neutrophils and when LM-CL is negligible [9]. Here we test the hypothesis, that LC-CL reflects normal oxygen reducing activity of white blood cells proceeding steadily at least in venous extravased blood. We also demonstrate that both LC-CL and LM-CL in whole blood may be stimulated by very weak photon fluxes and by carbon monoxide added to blood in tiny quantities.
Materials and methods
Blood of healthy volunteers or of patients with different pathologies was obtained by venous or finger puncture and stabilized with 3,2% sodium citrate (9:1). Blood was used in most cases 1 hour after it has been taken. All reagents unless otherwise specified were obtained from Sigma Chemical Co., USA. Stock solutions (10-1 M) of luminol were prepared in analytical grade dimethyl sulfoxide. It was diluted 50-fold in physiological saline (0.9% sodium chloride solution) just before use and added to a blood sample to a final concentration of 10-4 M. Stock solution (10-2 M) of lucigenin was prepared in saline. It was added to a blood sample to a final concentration of 10-4 M. RB in blood was stimulated by non-opsonized zymosan which was added to blood to a final concentration of 0,1 mg/ml. Carbon monoxide was obtained in the reaction of concentrated sulphuric acid with formic acid.
Neutrophils were isolated from blood using standard gradient centrifugation by Boyum’s method[10] with some modifications. Pure neutrophil suspensions contained 95-99% liveable cells (trypan blue test) and were used immediately after the isolation.
CL from blood was registered in liquid scintillation counters Mark-II (Nuclear-Chicago, USA), equipped with photomultipliers EMI 9750QB/1 in the mode of single photon counting (out-of-coincidences mode) in a tritium window. All measurements were performed at room temperature (19-21 oC). 1 ml Eppendorf polyethylene test tubes were used as blood containers. Dark counts with an empty test-tube in a counting chamber varied in the range of 50-60 counts/sec. All the operations were performed at dim ambient light illumination. In each experiment from 2 to 6 test tubes were filled with 0.2 ml of blood of the same donor. Half of the test tubes were completely wrapped with screens of aluminium foil and another half served as a control. After addition of zymosan and either of CL sensitises to blood all the samples in each experiment were counted in rotation unless otherwise noted. At different time points after the beginning of the experiment screens were removed from the experimental samples and CL detection was continued. In another experimental set-up for the studies of the effects of back reflected photons upon the cells, Eppendorf test tubes with blood were alternatively fixed either in empty glass vials, or in vials filled with water. In the former case some photons emitted from blood were reflected by the inner glass wall of a vial and returned back to blood sample installed in the optical focus. In the latter case count rate was about 15% higher than in the former due to the so called immersion effect of water (since refractive index of water is much more close to the refractive index of glass than that of the air, more light leaves the system filled with air).
The ability of neutrophils to reduce nitro-blue tetrasolium (NBT), expressed as percent reducing neutrophils was evaluated by a common method [11]. In brief an aliquot of blood (50 mkl) was taken from a blood sample, mixed with 50 mkl of 0,1% NBT solution in 0,15 M Na-P buffer (pH 7,2), the mixture was incubated at 37 oC for 20 min and at 20 oC for 20 min. Blood was smeared on a slide, dried out, fixed with methanol for 10 min. Slides were dyed with methylene green. 100 neutrophils were counted at each slide. NBT test was considered positive when dark blue diformasan granules (the product of NBT reduction) occupied from 5 to 100% of neutrophils cytoplasm. All the cells were grouped in 6 ranks according to their activity: range 1 -- 0%, range 2 -- 5-7%, range 3-- up to 30%, range 4 -- 30-50%, range 5 -- 50-90%, range 6 -- 100% of cytoplasm is filled with reduced diformasan granules.
Results
As we reported earlier, blood of different individuals differed in the rate of LM-CL development and its maximal intensity after RB initiation in it with zymosan. In particular, addition of luminol to blood of angina pectoris patients results in a rapid elevation of LM-CL intensity even in the absence of zymosan 8. Zymosan induces gradual elevation of LM-CL intensity in fresh donors’ blood followed with a very slow (up to 24 hours) relaxation, while in some patients’ blood addition of zymosan induces much more rapid escalation of LM-CL intensity and more rapid relaxation of it 9.
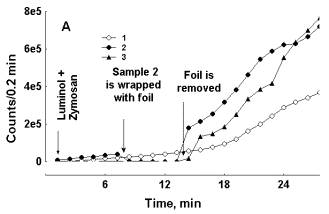
As it is demonstrated in Figure 1 (A and B), kinetics of LM-CL development during RB induced by zymosan was different in test tubes with blood covered with aluminium foil and in control test tubes. The “sign” of the effect: enhancement or attenuation of CL intensity in experimental test tubes after the foil cover was moved away from the test tube depended upon the rate of CL development in blood. If CL was accelerating slowly (count rate in the control samples did not exceed 100 000 counts/0.2 min at the moment of foil removal) CL intensity in the initially screened sample just after a screen removal usually exceeded that of a control one 1.5-5 fold and continued to increase faster that in control samples (Fig. 1A). On the contrary, at high rates of CL development in control blood samples count rate in such samples covered with foil either was not modified or was lower than in control samples at the moment when the screen foil was removed from the experimental samples (Fig. 1B).
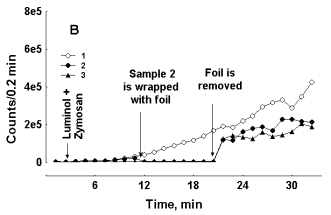
LC-CL readily develops in fresh blood even without the addition of RB initiators (Figure 2). It can be seen effects of aluminium foil on LC-CL development are generally the same as those observed with LM-CL development after RB inducing in blood. In the sample with slow development of LC-CL screening accelerates photon emission (Fig. 2, A), and in a blood preparation with fast development of LC-CL its inhibition is observed (Fig. 2, B).
|
|
|
Figure 1. Effect of aluminium foil screen over the test tube with blood upon luminol-dependent CL development in zymosan treated preparations of blood with slow (A) and fast (B) CL development. Curve 1 – control test tube, curve 2 -- test tube was wrapped with foil at the moment indicated with the arrow, curve 3 -- test tube was wrapped with foil before luminol and zymosan addition. In each experiment a preparation of blood was distributed in 3 equal portions counted in the mode of rotation (in turns).
|
|
|
Figure 2. Effect of aluminum foil screen on LC-CL development in two preparations of blood with slow (A) and fast (B) CL development. Curve 1 – control test tube, curves 2-4 -- test tubes were wrapped with foil before lucigenin addition. Foil was removed from respective test tubes at moments indicated by arrows. In each experiment a preparation of blood was distributed in equal portions among 4 samples, which were counted in the mode of rotation. Note the difference in ordinate scale values in A and B.
NBT test revealed the effect of foil screens upon activity of neutrophils in blood where RB was induced with zymosan and in which LC-CL was registered. In this experiment LC-CL was prominently higher after foil removal from the experimental sample than in the control sample (data not shown). It can be seen in the Figure 3 that after zymosan addition to blood the number of active neutrophils significantly increased in both samples. However, the total number of active cells in the sample screened with foil is larger than in the control sample (39% vs. 30%). It is notable that the number of cells belonging to high ranks (more than 50% of cytoplasm is filled with diformasan granules) is twice as more in the former sample in comparison to the control one (compare the bars ##5 and 6 in 1c and 1f). Thus, the NBT test gives an independent confirmation that sample screening with aluminium foil at the initial stage of RB development enhances reactive oxygen forms generation by neutrophils in whole non-diluted blood.
|
|
Figure 3. Effect of foil screening on neutrophil activity in whole blood evaluated by NBT test. 0c and 0f – distribution of neutrophils according to the ranges of their activity in a control and a foil screened samples just before the addition of zymosan and lucigenin to them.1c and 1f – same 6 minutes after addition of zymosan and lucigenin to the control and foil screened blood samples.
Technology of photon emission registering in liquid scintillation counters Mark 2 demands the usage of standard borosilicate glass vials as holders for samples with blood (Eppendorf test tubes). Test tubes were fixed in vials as it is shown in the upper right corner of Figure 4. In this experimental set-up some part of light emitted from blood samples can be reflected from the glass wall of a vial back to a sample. In order to examine this possibility we compared the level of radiation registered by PMT from test tubes with blood inserted in empty vials and in vials filled with water. These measurements has shown that CL intensity levels measured from the same sample of blood were 16 ± 4% (mean ± s. d.) higher when a vial was filled with water than when a test tube was inserted in an empty (containing air) vial. As the level of photon emission changed immediately after transfers of test tubes with blood from an empty vial to a water filled one and back, we concluded, that an increased light emission from water filled vials is due to lack of photon reflection from the inner wall of the vial back to its source due to the immersion effect of water.
The effects of transfer of a sample with blood from an empty vial to a vial filled with water and back on LM-CL following RB induction are demonstrated in Figure 4. When the sample was transferred to a vial filled with water at the initial stage of CL development its intensity initially jumped by 16% due to the immersion effect and then its acceleration temporarily retarded (Fig. 4, A). Sharp leaps of CL intensity were observed on sample transfers at the quasi-stationary stage of the process (Fig. 4, B).
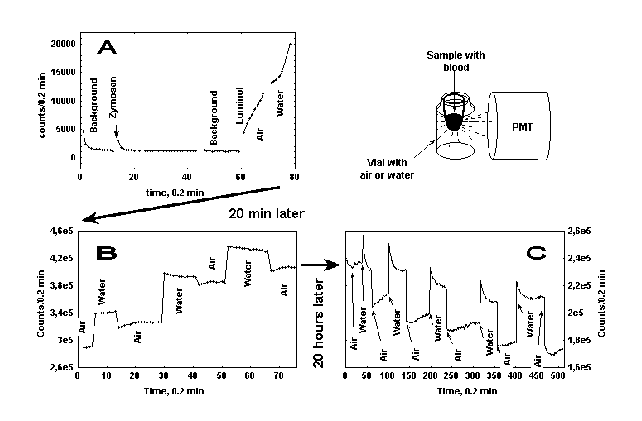
Figure 4: Effect of partial light reflection from the inner surface of the glass vial upon LM-CL at different stages of RB. Upper right corner: experimental set-up. An Eppendorf test tube with blood is inserted by turns into either empty or water filled glass vials. A – the initial stage of RB; B – the stationary stage of RB; C – the stage of RB decay. Upper right corner – experimental setup.
After a few minutes of the sample stay in a water-filled vial the stationary level of LM-CL in it was increasing when it was transferred back to an empty vial. On the other hand, when sample transfers from an empty to a water-filled vial and back were made at the stage of CL decay (Fig. 4, C), CL intensity in a water-filled vial was rapidly declining. After the sample was transferred back to an empty vial it appeared initially at a much lower value than previously, but began to rise up. These effects demonstrate again that irradiation of blood samples by back reflected photons affects the development of photon emission from blood. Lack of self-irradiation depresses photon emission from blood at relatively low levels of CL (at the stage of CL development and even more prominently at the stage of its decay). On the other hand, at high levels of photon emission (stationary stage) back reflected photons seem to depress CL (Fig. 4, B).
Effects of back reflected photons on CL from blood may have several explanations, though all of them should take into consideration the existence of a very strong amplifying mechanism in blood transforming energy of a small number of absorbed photons into a strong metabolic reaction of blood. Basing on our previous observations, that LC-CL in blood practically does not depend upon air access to blood, but rather upon oxygen supplied by erythrocytes present in high concentration in whole non-diluted blood 9 we suggested, that the effect of blood irradiation was mediated by oxy-hemoglobin (HbO2) present in erythrocytes. In fact, concentration of Hb in erythrocytes is very high, reaching 40% w/v. In other words Hb is very tightly packed in red blood cells. As it is the major chromophore in blood responsible for light absorbing, it may be supposed that at such a close package of Hb molecules in erythrocytes even one photon absorbed by one Hb molecule should provide enough energy for collective excitation of many molecules of Hb. If these molecules are in the form of HbO2, a lot of oxygen should dissociate and provide additional oxygen supply for oxidative processes in blood.
To test this hypothesis we tried to test another way for the facilitated oxygen release from erythrocytes. It is well known that CO affinity to Hb is more than 300-fold higher than that of O2, and if CO binds to 1 heme, enough energy can release to facilitate the dissociation of O2 from multiple HbO2 complexes. If this is the case, addition of CO to whole blood may enhance oxygen release and CL intensity boost.
To assess this supposition one bubble of carbon monoxide (ca. 1 mkl) or of air was passed through 0,2 ml of whole non-diluted blood or through 0,2 ml of neutrophil suspension containing same quantity of these cells as in non-diluted blood. As it can be seen in Fig. 5 (A and B), addition of CO to blood results in rapid boost in LC-CL in it unlike addition of air. In Figure 5, B it can be seen that after blood dilution with physiological saline intensity of LC-CL immediately falls down. Previously we observed a similar effect of blood dilution upon LC-CL both in the case when its intensity was not stimulated with CO and interpreted it as indicating of close relationships between the cells responsible for reactive oxygen species, and hence, CL production, and erythrocytes 9.
In Figure 5, C, photon emission increases both in the case of CO and air bubbling through blood, but in this particular specimen of blood initial rise in LC-CL was more rapid than in specimens, where only CO induces enhancement of LC-CL. While in the specimen of blood presented in Figure 5, A, LC-CL intensity reached 2500-3000 counts/0,1 min within 3 min after LC addition to it, in the specimen of blood presented in Figure 5, C, it reached 25 000 counts/0,1 min within 2 min after LC addition. Here again we can see that depending upon initial acceleration of CL intensity the effect of an external factor upon it may either be exposed, or hidden. In general, a specific CO effect was consistently observed in blood of healthy donors, while in blood of patients, where initial acceleration of photon emission was much higher (asthma, diabetes) it was either weak or absent. The effect of CO bubbling through neutrophil suspension upon LC-CL was opposite to that observed in whole blood (Fig. 5, D). Here CO strongly attenuated LC-CL. Thus, the effects of carbon monoxide upon the processes followed with LC-CL were opposite in whole non-diluted blood and in pure neutrophil suspensions.
|
|
|
|
|
|
Figure 5: The effect of a CO bubble, passed through whole non-diluted blood (A-C) and neutrophil suspension (D) upon LC-CL. A – a comparison of effects of CO and air passed through whole blood with low initial rate of LC-CL elevation. B - effect of blood dilution with saline upon LC-CL stimulated after CO addition to blood. C – Effect of CO and air upon LC-CL intensity in blood with high initial rate of CL rise. D – effect of CO upon LC-CL in a pure neutrophil suspension (25 000 cells/0,1 ml).
Discussion
Results presented above show that if part of low level photon flux originating from luminol- and lucigenin-dependent CL following oxidative processes in whole non-diluted blood is returned back to blood it may modulate initial radiation from blood by a feedback mechanism. According to the NBT-test, the increase of CL intensity in blood due to its irradiation with back reflected photons correlates with the enhancement of neutrophil activity.
Non-diluted blood is a system with a very high optical density and the ratio of neutrophils to erythrocytes in it is approximately 1:1000, so the question arises as how these very low intensity photon fluxes may have any measurable effect upon neutrophil activity it. It should be noted that blood in our experiments was not actively oxygenated. Previously we had demonstrated that LC-CL in blood is strongly dependent upon the interaction of neutrophils and erythrocytes, and that LC-CL depends on oxygen supplied by erythrocytes 9. Taking into consideration that lmax for luminol emission is 427 nm and for lucigenin emission it is 470 nm, and that several hemoglobin absorption maximums are not far from this spectral region, hemoglobin may be excited by back reflected photons. It is interesting to speculate that oxyhemoglobin excitation could support oxygen release from erythrocytes resulting in easier substrate supply for generation of ROS by neutrophils and enhancement of CL intensity in blood.
However, these considerations cannot readily explain attenuation of CL intensity by back-reflected photons in blood preparations with initially high rate of CL intensity growth. In our previous studies we demonstrated, that the major part of light emission comes from the lateral surface of the red blood pillar rather than from plasma or from a thin layer of white cells on top of it. We speculated that high levels of photon emission from blood may be explained by the phenomenon of secondary emission, implying non-radiative transfer of electron excitation generated in the bulk of blood to acceptor sites that emit photons registered by a photomultiplier 7. Efficiency of such a process should depend upon the structural organization of the system in which it occurs. We observed that the high rate of photon emission development is characteristic of blood of patients with cardiovascular diseases and of healthy donors’ blood after its long storage and correlates with high oxygen reducing activity of neutrophils in such blood. It was also demonstrated that in such blood preparations intensity levels were very unstable and strongly oscillated even under weak external influences 9, 10. It can be supposed, that in such blood preparations hemoglobin is already highly excited for the reasons discussed below, and that auxiliary irradiation of blood with back reflected photons does not provide an additional stimulus for oxygen release. On the other hand, additional irradiation of blood with very weak photon fluxes originated from blood itself and reflected back to it, may play an organising role so that less energy is lost from it, and the photon flux from such blood registered by a photomultiplier is lower after the reflective screen removal than the control sample.
The hypothesis that reactive oxygen species generation by white blood cells is enhanced due to an increase in oxygen availability, ensuing the enhancement of photon emission from blood, is supported in the studies of the effect of carbon monoxide upon whole blood and isolated neutrophils. While CO sharply intensified LC-CL in non-diluted blood; it had much weaker effect in saline-diluted blood, and inhibited LC-CL in a neutrophil suspension. CO is known to bind to heme much more tightly than O2. In whole blood its major target is deoxy-Hb. If CO was binding to free heme sites without affecting oxygen binding to other Hb molecules, no enhancement of oxidative processes could be observed. In fact, sharp elevation of LC-CL after CO addition to blood is a strong indication of highly co-operative processes proceeding in blood. Presumably, high energy released in heme-CO reaction in erythrocytes induces accelerated oxygen release from many HbO2 molecules resulting in the similar effect upon CL from whole blood as that produced by blood self-irradiation. Inhibitory effect of CO upon LC-CL in neutrophil suspensions is most probably explained by its ability to bind to heme enzymes of neutrophils responsible for oxygen reduction[12]. It is interesting to note that similar to the lack of stimulatory effect of back reflected photons upon blood with high initial rate of LC-CL and LM-CL, CO also did not stimulate photon emission from such specimens of blood. Indeed, if the process of oxygen release from erythrocytes was already occurring at a high rate, CO was unlikely to produce any additional effect.
Our observation, that CO enhances photon emission from non-diluted blood with low initial level of LC-CL may be related to the growing evidence that this gas, produced in the organism by special microsomal enzymes, heme-oxygenase I and II, exerts important regulatory effects upon many cells and tissues, in particular, upon rheological properties of blood, and the cardio-vascular system performance [13], [14], [15]. In particular, it has been shown that in the nervous system – one of the most avid oxygen consumer – CO production increases in hypoxia[16]. In line with our observations, these CO effects may be explained by the function of CO as the natural instrument to enhance oxygen release from HbO2 in sites where oxygen is most urgently needed. On the other hand, increased oxygen availability for the cells reducing it by one-electron path is resulted in the enhancement of electron excited species production, and, hence, in the additional oxygen release due to hemoglobin excitation. Besides, an increased level of electron excitation of hemoglobin eases CO dissociation from CO-hemoglobin, and provides CO evacuation from blood in lung ventilation. Thus, a lot of positive and negative feedback links in whole non-diluted blood between different blood cells employing electron excited species, in particular, may provide for efficient supply of oxygen and other consumables to cells and tissues and also effective evacuation of waist products of tissue metabolism.
Whether these speculations are true or not, it is demonstrated here that even very low intensity light irradiation of blood can effectively influence important physiological processes in whole blood. This observation is in accord with recent claims that blood may mediate effects of ambient light upon the nervous system[17].
Acknowledgements
This work was supported by the Russian Scientific Research Foundation, grant 96-04-50232.
References
[1]. R. C. Allen, R. L. Strjrnholm, and R. H. Steele, “Evidence for the generation of an electronic excitation states (s) in human polymorphonuclear leukocytes and its participation in bactericidal activity,” Biochem. Biophys. Res. Commun., 47, pp. 679-684, 1972.
[2]. D. A. Kalbhen, “Introductory remarks on problems of chemiluminescence in liquid scintillation counting,” Liquid scintillation counting. Recent Application and Development. Eds. C.-T. Pehg, D. L. Horrocks, E. L. Alpen, Vol. 2, Sample preparation and application, Academic Press, New York, pp. 273-280, 1980.
[3]. R. C. Allen, “Lucigenin chemiluminescence: a new approach to the study of polymorphonuclear leukocyte redox activity”, Bioluminescence and Chemiluminescence: Basic Chemistry and Analytical Applications. Eds. M. A. DeLuca, W.D. McElroy, pp. 63-73, 1981.
[4]. R. C. Allen, “Phagocytic leucocyte oxygenation activities and chemiluminescence: A kinetic approach to analysis,” Methods in Enzymology, Bioluminescence and Chemiluminescence, Vol. 133, pp. 449-493, 1986.
[5]. G. Cilento, “Photobiochemistry without light”, Experientia, 44, pp. 572-576, 1988.
[6] C. Reid, “Excited states in chemistry and biology,” Butterworths Scientific Publications, London, Chapter 7, 1957.
[7]. V. L. Voeikov and C.N. Novikov, "Relative independence of luminol-enhanced intensity of photon emission during oxidative burst from nondiluted human blood on the volume and surface area of the sample", Optical Diagnostics of Biological Fluids and Advanced Techniques in Analytical Cytology, SPIE Proc., San Jose, CA, 2982, pp. 65-75, 1997.
[8]. V. L. Voeikov, C. N. Novikov, and N. I. Siuch, "Alterations in luminol-enhanced chemiluminescence from nondiluted whole blood in the course of low-level laser therapy of angina pectoris patients", Ultrasensitive Biochemical Diagnostics II”, SPIE Proc., San Jose, CA, USA, 2985, pp.286-294, 1997.
[9] V.L.Voeikov, C.N. Novikov, N.D Vilenskaya. “Low-Level Chemiluminescent Analysis of Nondiluted Human Blood Reveals its Dynamic System Properties”. Journal of Biomedical Optics. v. 4, pp.54-60, 1999.
[10]. A. Boyum “Isolation of leucocytes from human blood. A two-phase system for removal of red cells with methylcellulose as erythrocyte-aggregating agent”. Scand. J. Clin. Lab. Invest. Suppl.; 97, pp. 9-29, 1968.
[11]. B. H Park, S. M. Fikrig, and E. M. Smithwick, “Infection and nitroblue-tetrazolium reduction by neutrophils. A diagnostic acid”, Lancet, 2, pp. 532-534, 1968
[12]. H. Sumimoto, K. Takeshige, H. Sakai, S. Minukami. “A cell-free preparaion of human neutrophils catalyzing NADPH- dependent conversion of leukotriene B4.” Biochem. Biophys. Res. Commun., v. 125., p. 615-621, 1984.
[13]. P.J. Downard, M.A. Wilson, D.A. Spain, P.J. Matheson, Y. Siow, R.N. Garrison. “Heme oxygenase-dependent carbon monoxide production is a hepatic adaptive response to sepsis.” J. Surg. Res. v. 15, pp. 7-12, 1997
[14]. R. Henningsson, P. Alm, P. Ekstrom, I. Lundquist. “Heme oxygenase and carbon monoxide: regulatory roles in islet hormone release: a biochemical, immunohistochemical, and confocal microscopic study.” Diabetes, v.48, pp. 66-76, 1999.
[15]. R.A. Johnson, E. Colombari, D.S. Colombari, M. Lavesa, W.T. Talman, A. Nasjletti. “Role of endogenous carbon monoxide in central regulation of arterial pressure.” Hypertension, v. 4, pp. 962-967, 1997.
[16]. T.M. Dawson; S.H. Snyder. “Gases as biological messengers: nitric oxide and carbon monoxide in the brain.” J. Neurosci, v. 9, pp. 5147-5159, 1994
[17]. D.A. Oren, M.Terman. “Enhanced: tweaking the human circadian clock with light”, Science, v. 279, pp. 333-334, 1998.