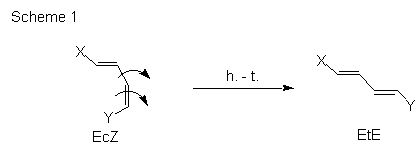
| Site home page | Conference home page | Discussion |
Does Hula-Twist Contribute to Z/E Isomerization in Solvents?
Brief Notes on the Hula-Twist Mechanism.
Olga Dmitrenko* and Wolfgang Reischl"
*Institute of Surface Chemistry, National Academy of Sciences of Ukraine, 252022 Kiev, Ukraine (current address: Department of Chemistry and Biochemistry, Brown Laboratory, University of Delaware, DE 19716, USA, odmitr@udel.edu)
"Department of Organic Chemistry, University of Vienna, A-1090 Vienna, Austria
Introduction.
The hula twist mechanism (h.-t.) has been postulated by R. S. H. Liu for the light driven Z to E ismerization of 11-cis-retinal to all-trans-retinal bound to opsin via a Schiff-base [1]. This mechanism allows the movement of substituents attached to the double bond in a more volume-saving manner (Scheme 1) than the ordinary mechanism. Few years ago, W. Fuss's group provided new experimental support to this mechanism by low-temperature studies on previtamin D photochemistry [2].
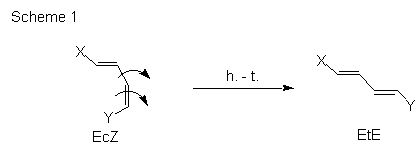
Despite of this success the hula-twist mechanism is still a subject of debates [3]. In brief, there is the hypothesis that hula-twist is a mechanism of double bond isomerization dictated by volume-saving factors in restricted media.
In this poster we will briefly address the questions if the hula-twist mechanism is a general phenomenon for Z/E isomerization and can it occur without volume restrictions in solvents.
Experimental facts.
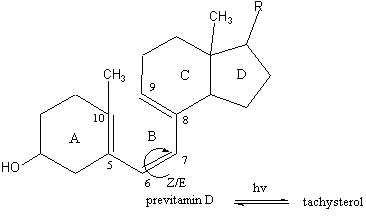
1. It has been demonstrated by Dauben et al. that the quantum yield of previtamin D3 Z/E isomerization depends on wavelengths of irradiation. More specifically: there is a sudden decrease at wavelengths between 298 and 303 nm [4].
2. In a later paper [5], no similar behavior was found in the wavelength dependence of Z/E quantum yields for the two model compounds 1a and 2a:
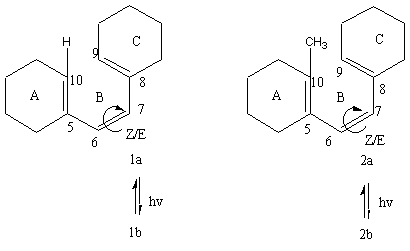
Experimental Z/E data are plotted in Figure 1. In cases of 1a and 2a, both dependencies are almost linear which is not the case for the previtamin D3 Z/E quantum yield dependence.
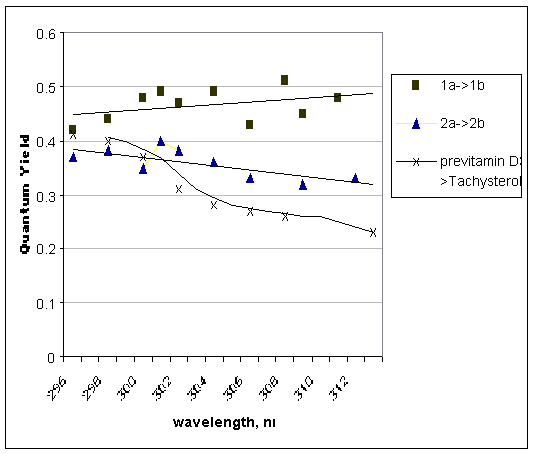
Figure 1. Z/E photoisomerization quantum yield vs irradiation wavelengths measured in ether. 1b and 2b are E isomers of 1a and 2a, respectively. Previtamin D3 data are from [4], data for 1a and 2a are from [5].
Conclusion: Previtamin D3 has apparently a difference in photobehavior. The origin of this difference is caused by the different structural features of previtamin D, 1a and 2a.
Conformational analysis. Ground state conformations have been optimized for 1a, 2a and 3-desoxy-previtamin D models at B3LYP/6-31G(d) level of theory [6]. In order to reduce the number of time-consuming optimizations only models with 3b-hydrogen of the first ring in the equatorial position (equivalent to previtamin D in its OH-equatorial A-ring half-chair conformation) have been calculated. The second C-ring in 1a and 2a was allowed to adopt both half-chair conformations. The results are summarized in Table 1:
Table 1. Estimated conformational population of 1a (R=H), 2a (R=CH3) and 3-desoxy-previtamin D (R=CH3) model compounds based upon B3LYP/6-31G(d) calculations.
|
Z-isomer |
|
|
|
1a |
2.4 % |
97.6 % |
|
2a |
8.4 % |
91.6 % |
|
3-desoxy-Previtamin D |
25.9 % |
74.1 % |
The increase of folded cZc conformations in order of 1a<2a<previtamin D is a result of complex network of steric repulsions and, in 2a and previtamin D, CH/p bonding interactions between methyl group and C6=C7 double bond. We have demonstrated recently [7] that these CH/p interactions are dictating contributors to the conformational equilibrium of previtamin D. Thus, the increase of cZc population with presence of methyl group at C10 was a logical expectation for the comparative conformational studies.
Conclusion:
The observed specificity of previtamin D Z/E photoisomerization is due to substantial amount of folded cZc conformations in the ground-state mixture.
Modeling. In low-temperature studies of provitamin D photoisomerization [2] it has been proposed that cZc-previtamin D performs Z/E isomerization via hula-twist mode of motion. The reaction was conducted in a cold matrix, and no appreciable activation barrier was observed. The conclusion of the authors was that "the isomerization can also occur at higher temperatures and outside of a matrix"[2].
|
|
Hula-twist motion in previtamin D: C6-C7 has single-bond character at the S1 excited state and one may expect to have a tendency for dihedral angle around it to be 90°. C5-C6 is less "flexible" at the excited surface, rotation around it will lead to a transition state structure with a certain barrier. If to denote by p and P non-planar geometries for single and double bond rotations, respectively, the excited state process can be described by the following expression: cZc * à {cPc* ---> pPc*} àconical intersection |
Thus, in solvent at short-wavelength irradiation (when there is enough energy to overcome the small hula-twist barrier [2] ) one may assume that cZc conformers perform ring-closure and hula-twist Z/E passage with equal probability. At long-wavelength irradiation, cZc conformers do not posses sufficient energy to pass the hula-twist barrier and thereby relaxing by ring-closure.
Kinetic and spectral modeling based upon above assumption resulted in an excellent agreement with the experimental dependence of the cis-trans (Z/E)/ring-closure ratio of quantum yields (Figure 2A).
Previous modeling [8] based upon idea that cZc are precursors for ring-closure only and tZc are precursors for cis-trans (Z/E) isomerization (NEER hypothesis in its traditional form [9]) lead to a good agreement only at the long-wavelength region [8] (see Figure 2B for a comparison).
(A): 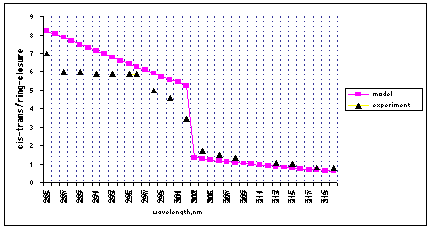
(B):
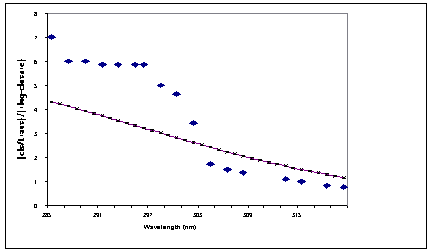
Figure 2. A comparison of calculated quantum yield
values for ![]() with experimental values for
with experimental values for
![]() (data are taken from ref. [4]).
(A): calculated with the assumption that cZc conformers
contribute to cis/trans isomerization equally as to ring-closure at l<302 nm; (B): calculated according conformational control principle:
cZc conformers are only precursors for ring-closure reactions, whereas tZc conformers
lead to cis-trans isomerization.
(data are taken from ref. [4]).
(A): calculated with the assumption that cZc conformers
contribute to cis/trans isomerization equally as to ring-closure at l<302 nm; (B): calculated according conformational control principle:
cZc conformers are only precursors for ring-closure reactions, whereas tZc conformers
lead to cis-trans isomerization.
Conclusion.
Our modeling of data on the Z to E isomerization in previtamin D and related model compounds found in the literature indeed suggest that the hula twist mechanism for photochemical double bond isomerization may occur not only in restricted matrices but also in solutions. This suggestion is in agreement with thinking that hula-twist "probably corresponds to the normal path of cis-trans isomerization" [2].
Acknowledgment. This work was supported by the Osterreichische Nationalbank (Jubiläumsfondsprojekt Nr.: 7395 to W. R.). We also thank the University of Vienna Computer Center for generous amounts of computer time.
References.
1. (a) R. S. H. Liu, D. T. Browne, (1986) A Bioorganic View of the Chemistry of Vision: H.T.-n and B.P-n,m Mechanism for Reactions of Confined, Anchored Polyenes, Acc. Chem. Res. 19 , 42; (b) R. S. H. Liu, A. E. Asato, (1985) Photochemistry of Polyenes 22. The Primary Process of Vision and the Structure of Bathorhodopsin - A Model Study, Proc. Natl. Acad. Sci. USA 82, 259-263; (c) K. Schulten, P. Tavan, (1978) A Mechanism for the Light-Driven Proton Pump of Halobacterium halobium, Nature 272, 85; (d) R. S. H. Liu, D. Mead, A.E. Asato, (1985) Application of the H.T.-n Mechanism of Photoismerization to the Photocycle of Bacteriorhodopsin: A Model Study, J. Am. Chem. Soc. 107, 6609; (e) R. H. S. Liu (1998) Looking Back to My Research Effort (1962-1998) www.bgsu.edu/departments/photochem/; Spectrum 11(4), 7
2. A. M. Muller, S. Lochbrunner, W. E. Schmid, W. Fuss, (1998) Low-Temperature Photochemistry of Previtamin D: A Hula-Twist Isomerization of a Triene, Angew. Chem. Int. Ed. 37, 505.
3. as indicated by announced lecture in the preliminary program of Pacifichem 2000 "Revisiting hula-twist: is it a general mechanism for photoisomerization? Does it violate the NEER hypothesis?" by Liu and Hammond.
4.W. G. Dauben, B. Disanayaka, D. J. H. Funhoff, B. E. Kohler, D. E. Schilke, B. Zhou, J. Am. Chem. Soc. 113 (1991) 8367.
5. W. G. Dauben, B. L. Zhou, J. Y. L. Lam, Photochemistry of structural analogues of previtamin D3: Generality of the wavelength-dependent triene photocyclization. J. Org. Chem. 62 (1997) 9005.
6. M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Cioslowski, J. V. Ortiz, A. G. Baboul, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W Gill, B. Johnson, W. Chen, M. W. Wong, J. L. Andres, C. Gonzalez, M. Head-Gordon, E. S. Replogle, J. A. Pople, Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh PA, 1998; (b) A. D. Becke, Phys. Rev. A 37 (1988) 3098; C. Lee, W. Yang, R. G. Parr, Phys. Rev. B41 (1988) 785; A. D. Becke, J. Chem. Phys. 98 (1993) 5648; P. J. Stevens, F. J. Devlin, C. F. Chablowski, M. J. Frisch, J. Phys. Chem. 80 (1994) 11623.
7. (a) O. Dmitrenko, J.H. Frederick, W. Reischl. Ab Initio Calculations on Previtamin D, Vitamin D and their Simplest Models, W. J.Mol.Struc.(Theochem), 530 (2000) 85-96
(b) O. Dmitrenko, J.T. Vivian, W. Reischl, J.H. Frederick, Theoretical Studies of the First Strongly Allowed Singlet States of 3-Desoxy Analogs of Previtamin D, Vitamin D and Their E-Isomers, J.Mol.Struc.(Theochem), 467 (1999) 195-210
8. (a) O. Dmitrenko, J. H. Frederick and W. Reischl, Previtamin D conformations and the wavelength-dependent photoconversions of previtamin D, submitted to J.Photochem.Photobiol.A:Chem; (b) Previtamin D Conformations And Estimation On Their Role In The Wavelength Dependence of Previtamin D Photosynthesis In Vitro, O. Dmitrenko, J. H. Frederick, W. Reischl, - poster contribution to Second Internet Conference on Photochemistry and Photobiology. July 16-Sept 7 1999.
http://www.photobiology.com/photobiology99/contrib/olga/index.htm
9. (a) H. J. C. Jacobs and E. Havinga, Adv. Photochem. 11 (1979) 305. (a) H. J. C. Jacobs, J. W. H. Gielen, E. Havinga, Tetrahedron Lett. 22 (1981) 4013; (b) A. M. Brouwer, J. Cornelisse, H.J.C. Jacobs, Tetrahedron 43 (1987) 435; (c) H. J. C. Jacobs, Pure & Appl. Chem. 67 (1995) 63.