| Site home page | Conference home page | Discussion |
Theoretical Insight On The Urocanic Acid E/Z Photoisomerization Wavelengths Dependence
Olga Dmitrenko2*and Wolfgang ReischlE
odmitr@udel.edu
2 Institute of Surface Chemistry, National Academy of Ukraine, Kiev 252022, Ukraine
(current address: Department of Chemistry and Biochemistry, Brown Laboratory,
University of Delaware, DE 19716, USA)
EInstitute of Organic Chemistry, University of Vienna, A-1090 Vienna, Austria
Abstract. B3LYP/6-31+G(d,p) conformational analysis has been performed on urocanic acid (UA, 1), methyl urocanate (Me-UA ,2) and anionic and zwitterionic forms (3, 4) of UA. Of the eight most stable conformers of trans-UA (E-UA), the s-trans, s-cis conformation of E-UA (tEc-UA) is the global minimum. No marked differences between the conformational distribution of undissociated UA and Me-UA conformers have been found. However zwitterionic UA was calculated to exist as a mixture of two non-planar conformers with strong preference to its tE geometry. CIS calculation results on the excited state energies of planar and non-planar zwitterionic UA structures indicate that deviation from planarity may result in the disappearance of the "dark" singlet state below the strongly allowed π-π* transition. It has been assumed that at the ground state, both planar and non-planar geometries of zwitterionic UA are dynamically existing due to shallow minima and may contribute alternatively in excitation of different states upon short and long wavelength irridation. Short wavelengths excitation of non-planar structures contribute to intersystem crossing, whereas planar conformers decay to the lower singlet state and perform the E/Z isomerization.
Introduction
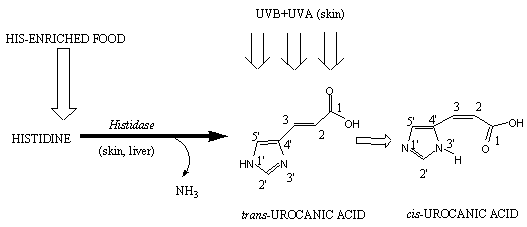
The product of the histidase reaction, trans-UA (Figure 1) is a major ultraviolet (UV) absorbing component in the human epidermis [1]. It has been demonstrated that its photochemical E/Z isomerization into cis-UA [2] controls a number of biological responses in living organisms to UV exposure [1-3 and references therein]. This makes a mechanistic understanding of the underlying photo-physical and photo-chemical processes of significant interest for the understanding of urocanic acid´s biological mode of action.

Figure 1. Initial key processes of histidine metabolism related to urocanic acid.
This work is stimulated by our general mechanistic interest in epidermal photoreactions. In particular the formation of previtamin D (the thermal precursor of vitamin D) from 7-dehydrocholesterol [4a]. The initial step of vitamin D synthesis involves an epidermal photoreaction leading to the accumulation of previtamin D [4]. It has been found that ring-closure and Z/E photoisomerization reactions depend on irradiation wavelength [4b] and the mechanism of this dependence is still unclear [4a]. Similar photochemical behavior is observed for UA [5]: its E/Z isomerization in vitro has a strong wavelength-dependence. Naturally, it is of interest to know if there is a common underlying mechanism controlling the wavelength-dependent photochemistry of both epidermal molecules.
Studies show that the quantum yield for E/Z isomerization strongly increases with wavelength [5a,b]. However, the methyl ester of UA (Me-UA) shows an opposite behavior (Table 1). Recent studies on some urocanic acid derivatives [6c] demonstrated that replacing the carboxyl hydrogen with CH3, C2H5 and C12H25 does shift E/Z isomerization efficiency to shorter wavelength. Thus, the nature of the carboxyl group (free acid or ester) in UA strongly determines its specific wavelength-dependent performance.
Table 1. Urocanic Acid (UA) and Metyl Urocanate (Me-UA) E/Z Isomerization Quantum Yields at Different Wavelengths.
|
wavelength, nm |
fE/Z (UA) a) |
fE/Z (Me-UA)b) |
|
254 |
0.043; 0.058 |
0.94 |
|
266 |
- |
0.84 |
|
290 |
0.079 |
0.78 |
|
313 |
0.49; 0.52 |
0.67 |
a) data are from [5], aqueous solutions; b) data are from [6], acetonitrile solutions.
Comparison of UV absorption data summarized in Table 2 does not provide any explanation to this fact. Moreover, UA in aqueous solutions at pH 6.4 and lower, has a blue shifted maximum of absorption that should provide the best spectral conditions for E-UA activation at shorter wavelengths region.
Table 2. Summary of Experimental Data on UV-Absorption Maxima of trans-UA
|
Solvent |
Ref. |
UA form |
lmax, nm |
|
aqueous solution pH 5.6 |
[5c,d] |
zwitterionic |
268 |
|
aqueous solutions pH 6.4 |
[7] |
zwitterionic |
264, shoulder at 225 |
|
aqueous solution pH 11.0 |
[7] |
anionic |
279, shoulder at 228 |
|
aqueous solution pH 7.2 |
[5c,d],[8] |
anionic |
277, 278 |
|
water, acetonitrile |
[6a] |
Me-UA |
288 |
|
CH2Cl2 |
[6a,b] |
Me-UA |
284, 286 |
This puzzling photobehavior of UA attracted much computational efforts [9]. The global minimum geometry optimization were performed using semi-empirical, AM1, PM3, MNDO and HF/3-21G and MP2/6-31G(d) ab initio methods [9a,b]. Complete active space SCF(CASSCF) ab initio calculations were performed on the singlet ground state and the triplet and the singlet manifolds of the lowest-lying π --> π* (HOMO --> LUMO) excitation of the neutral and the anionic forms of UA in [9c]. Based upon a kinetic model of Laihia et al.[10] , Lahti and co-authors proposed a model in which the trans-to-cis isomerization proceeds via both the triplet and the singlet manifolds in anionic UA.
In [9d], Olivucci et al. have presented a multiconfigurational second-order perturbation theory (CASPT2) study of the lowest lying states in the gas-phase electronic spectra of trans- and cis-urocanic acid. For their geometry optimizations they used MP2/6-31G(d) and CASSCF/ANO-L(4s3p1d,2s) levels of theory. It was found for trans-UA that the π - π * and n(O)- π * surfaces cross at a coordinate between that of the ground-state structure and the equilibrium excited state structure. This provides useful insight into the wavelengths dependence of UA isomerization as a consequence of the presence of multiple electronic states. However, the potential role of the triplet state is not considered in this study despite of the experimental evidence of its involvement [5c-d].
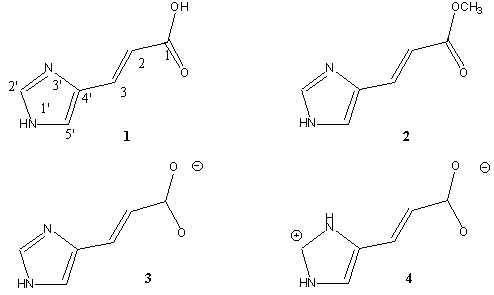
In order to clarify the role of the nature of the carboxyl group in urocanic acid photoisomerization, we performed density functional studies on the ground states of UA (1), Me-UA (2) and UA anionic- and zwitterionic forms 3, 4 (zwUA) and excited states calculation based on that geometries.
Computational details
The calculations were performed with the Gaussian 98 program package [11]. The Becke three-parameter hybrid functional [12a, 13a] combined with the Lee, Yang and Parr (LYP) correlation functional [12b], denoted B3LYP [13b], was employed in the calculations using density functional theory (DFT). Geometries were optimized with the 6-31G+(d,p) basis set [11]. The stationary points on the potential energy surfaces were characterized by frequency calculations. The partial charges were calculated using the NBO method implemented in the Gaussian 98 program [11e]. Solvent effect on anionic form of UA have been probed using the SCIPCM [14] model with the B3LYP/6-31G(d) basis set and a dielectric constant e= 78.9. Electronic excited states were calculated using the single-excitation configuration interaction method (CIS) [15] with 6-31+G(d,p) basis set on the B3LYP/6-31+G(d,p) optimized structures.
Throughout the text, bond lengths are in Ångstroms and bond angles are in degrees.
Results and discussion
The eight most stable conformers of trans-UA (E-UA) and their relative energies at B3LYP/6-31+G(d,p) level of theory are shown in Figure 2. In agreement with earlier published results [9], our optimizations confirm that s-trans, s-cis conformation of E-UA (tEc-UA) is the global minimum at the ground state surface. The next minimum, tEc-TautUA (tautomeric form), is slightly higher in energy (0.4 kcal/mol).
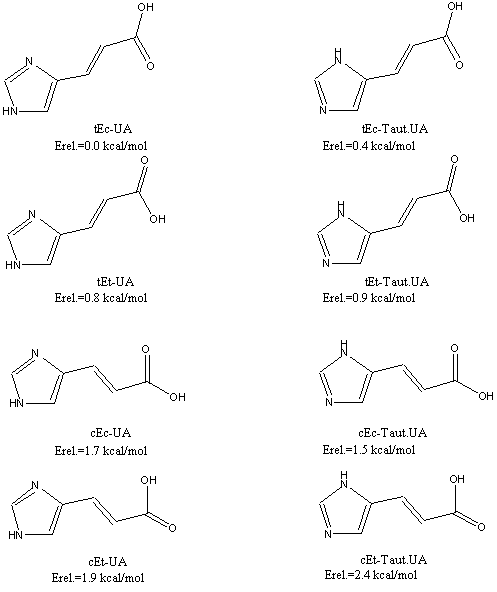
Figure 2 Conformations of UA and their relative energies calculated at B3LYP/6-31+G(d,p) level of theory. All structures are planar.
Chemical modification, charge development or solvation does affect the conformational distribution of compounds with flexible single bonds usually. Surprisingly, when comparing the calculated data for neutral UA and Me-UA (Table 3) no marked difference in the conformational stability and distribution is found. However, a large solvent effect and a significant change in the conformer population were obtained for the anionic form of UA indicating a strong effect of the charged carboxyl group.
Table 3. Relative conformational energies (kcal/mol) of UA, Me-UA and UA-anion (B3LYP/6-31+G(d,p)).
|
Geometry |
UA |
Me-UA |
UA-anion |
|
cEc |
1.7 |
1.7 |
8.1 (1.8)a |
|
cEt |
1.9 |
2.1 |
|
|
tEc |
0.0 |
0.0 |
8.4 (0.8) |
|
tEt |
0.8 |
1.1 |
|
|
Taut-cEc |
1.5 |
1.3 |
2.1 (0.6) |
|
Taut-cEt |
2.4 |
2.6 |
|
|
Taut-tEc |
0.4 |
0.3 |
0.0 (0.0) |
|
Taut-tEt |
0.9 |
1.0 |
aResults of SCIPCM (e=78.4) solvent modeling (B3LYP/6-31G(d)//HF/6-31+G(d))
The most stable conformers of zwitterionic UA are shown in Figure 3. We found that both conformers posses a non-planar structure at both B3LYP and MP2 levels of theory whereas planar geometry represent a saddle point on the ground state surface. According to the calculated NBO charge distribution (Table 4), one may conclude that there is an electrostatic attraction and tendency to minimize charge separation in balance with steric interactions that leads to non-planar zwitterionic minima. Since there is no intra molecular charge separation in the neutral and anionic forms of UA, a planar geometry for their ground state conformers is highly favored. Our data on CIS excited state calculations indicate that deviation from planarity causes significant changes in the electronic states ordering (Table 5).
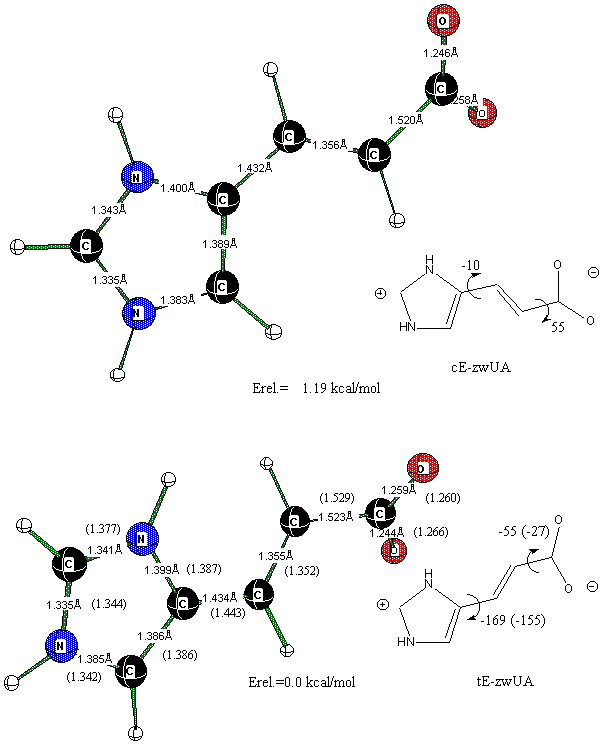
Figure 3. Zwitterionic structures of UA optimized at B3LYP/6-31+G(d,p). Numbers in parenthesis are at the MP2/6-31+G(d) level of theory.
Table 4. NBO charges for functional units of neutral, anionic and zwitterionic urocanic acid (calculated with MP2 density and 6-31+G(d,p) basis set).
|
unit |
tEc-UA |
tE-UA-anion |
planar tE-zwUA a |
non-planar tE-zwUA |
|
5-membered ring b |
0.026 |
-0.085 |
0.792 |
0.774 |
|
-CH=CH- |
0.026 |
-0.070 |
-0.038 |
-0.056 |
|
COO(H) b |
-0.052 |
-0.845 |
-0.754 |
-0.719 |
a transition structure (one imaginary frequency); b the charge on hydrogen is included according to its presence in the corresponding structure.
Table 5. Results of CIS(NStates=6, 50-50)/6-31+G(d,p) calculations on optimized tEcUA (1) and tE-UA anion (3).
|
State |
E, eV |
l, nm |
osc.str. |
State |
E, eV |
l, nm |
osc.str. |
|
tEcUAa |
tE-UA anion (COO-)b |
||||||
|
T1 |
2.6434 |
450.79 |
0.00 |
T1 |
2.7616 |
448.95 |
0.00 |
|
T2 |
4.2281 |
293.24 |
0.00 |
T2 |
4.0507 |
306.08 |
0.00 |
|
T3 |
4.9615 |
249.89 |
0.00 |
T3 |
4.6267 |
267.97 |
0.00 |
|
S1 |
5.4973 |
225.53 |
0.91 |
S1 |
4.6943 |
264.12 |
0.00 |
|
T4 |
5.8979 |
210.22 |
0.00 |
T4 |
5.0812 |
244.01 |
0.00 |
|
T5 |
5.9353 |
208.89 |
0.00 |
T5 |
5.2335 |
236.90 |
0.00 |
|
S2 |
6.0374 |
205.36 |
0.00 |
T6 |
5.3494 |
231.77 |
0.00 |
|
T6 |
6.0726 |
204.17 |
0.00 |
S2 |
5.5239 |
224.45 |
0.01 |
|
T7 |
6.3318 |
195.81 |
0.00 |
S3 |
5.6112 |
220.96 |
0.17 |
|
S3 |
6.4247 |
192.98 |
0.00 |
S4 |
5.8824 |
210.77 |
0.00 |
|
T8 |
6.5950 |
188.00 |
0.00 |
S5 |
5.9171 |
209.53 |
0.73 |
|
S4 |
6.6356 |
186.85 |
0.16 |
S6 |
5.9233 |
209.32 |
0.00 |
aB3LYP/6-31+G(d,p) total energy is -492.2372839 a.u.
bB3LYP/6-31+G(d,p) total energy is -491.6726428 a.u., proton affinity PA=354.32 kcal/mol
Table 6. Results of CIS(NStates=6, 50-50)/6-31+G(d,p) calculations on the optimized (B3LYP/6-31+G(d,p)) planar (saddle point of first order) and non-planar (minimum) tE-zwUA.
|
State |
E, eV |
l, nm |
osc.str. |
State |
E, eV |
l, nm |
osc.str. |
|
planar tE-zwUA |
non-planar tE-zwUA |
||||||
|
T1 |
2.7504 |
450.79 |
0.00 |
T1 |
2.8700 |
432.00 |
0.00 |
|
T2 |
3.7331 |
332.12 |
0.00 |
T2 |
3.6128 |
343.18 |
0.00 |
|
T3 |
4.3779 |
283.20 |
0.00 |
T3 |
4.6684 |
265.58 |
0.00 |
|
T4 |
4.3948 |
282.11 |
0.00 |
T4 |
5.0106 |
247.44 |
0.00 |
|
S1 |
4.9451 |
250.72 |
0.00 |
T5 |
5.1101 |
242.62 |
0.00 |
|
T5 |
5.0349 |
246.25 |
0.00 |
S1 |
5.1910 |
238.84 |
0.50 |
|
T6 |
5.2828 |
234.69 |
0.00 |
S2 |
5.3793 |
230.48 |
0.00 |
|
S2 |
5.3213 |
232.99 |
0.51 |
T6 |
5.6003 |
221.39 |
0.00 |
|
S3 |
5.5635 |
222.85 |
0.00 |
S3 |
5.6755 |
218.46 |
0.00 |
|
S4 |
5.8722 |
211.14 |
0.00 |
S4 |
5.8951 |
210.32 |
0.30 |
|
S5 |
5.8874 |
210.59 |
0.02 |
S5 |
6.1145 |
202.77 |
0.06 |
|
S6 |
6.1353 |
202.08 |
0.50 |
S6 |
6.2010 |
199.94 |
0.01 |
The calculated (CIS) first singlet excitation energy for neutral tEc-UA, 5.49 eV (oscillator strength 0.91) (see Table 5), is in good agreement with π-π* transitions at 4.93 eV (oscillator strength 0.12) and 5.40 eV calculated at CASPT2 level in [11d]. In these calculations, first π-π* excited state has a significant contribution of doubly excited configurations (24% [11d]). Thus, CIS method has located one singlet excited state. Interestingly, for planar zw-UA we got much better agreement with CASPT2 data. The first allowed singlet state ("bright" state) in planar zw-UA (S2) is of the same energy as the π-π* transition (at 232 nm) calculated in [11c] (see Table 6). In non-planar zw-UA, this π-π* transition became to be first allowed singlet state (S1). Thus, from these comparative data, one may conclude that the deviation from planarity in non-planar tE-zwUA results in the re-ordering of the electronic states in such a way that the energy of the "dark" singlet state increases in non-planar structures and the "bright" singlet state become now the lowest. Based upon this observation one may assume that in non-planar structures, intersystem crossing is more probable due to the absence of "dark" singlet state below the excited singlet state S1. In view of this, the excited molecule may undergo isomerization through the initially excited singlet state or perform an intersystem crossing. Planar UA molecules may decay from "bright" S2 to lower "dark" singlet surface S1 and follow only the singlet isomerization pathway. Such a separation of excited pathway seems to be possible due to our recent studies on the triplet surface of trans-UA [16], which we believe to posses similar properties as the lowest singlet excited surface. We found two triplet state minima separated by a small barrier. One minimum is entirely a planar triplet tEc-UA, the other one has the former C2=C3 double bond twisted to 95.4° (Figure 4).
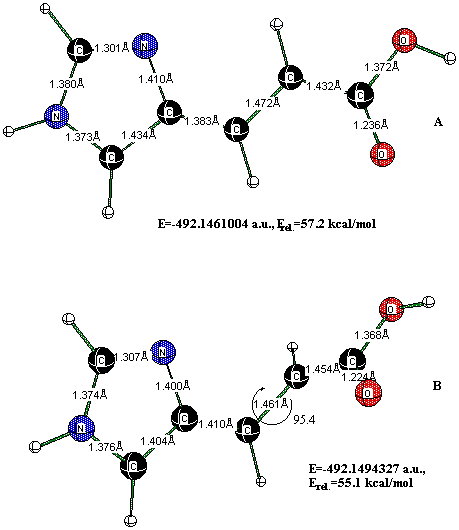
Figure 4. Triplet tEc- UA optimized at UB3LYP/6-31+G(d,p). Relative energies (Erel.) are given with respect to the ground state tEc-UA (1) conformer.
One may assume that excitation of planar and non-planar structures (neutral, anionic, zwiterionic) populate different basins, separated by barrier at the excited state surface. In some way, it is analogous to the case of excitation of different conformers, which can not interconvert at the excited state surface and follow their individual way of relaxation.
Naturally, a planar zw-UA, which is a transition structure, can not be considered as a real intermediate in the photochemical process. In order to attempt the mechanistic model based upon the above discussed difference in the excited states ordering, we have assumed that trans-UA due to dynamical deprotonation/protonation processes and very shallow minima in a solvent may perform a single-bonds twisting movement between planar and non-planar forms. In this case, high-energy photons excite non-planar structures (usually non-planarity results in blue-shifted absorption [6a]) and lead to both singlet (from initially excited state) and triplet relaxation pathways. While longer wavelength excitation of the planar structures results in isomerization via the singlet pathway.
The absorption spectra given in Table 2 provide an additional support to this idea. Me-UA has absorption maximum at longer wavelength then UA indicating a more planar chromophore which is in line with our prediction. According to our model, one may expect no activation of a triplet channel in planar Me-UA and, therefore only specific for trans-UA, a wavelength-dependent photochemistry. The absence of a wavelength effect in the Z/E isomerization of cis-UA, a strictly planar structure caused by the presence of an intramolecular hydrogen bond [9e], also supports our idea.
Conclusions
The most stable conformer of UA according to gas-phase ab initio calculations (HF and B3LYP, ours and in [9]) is tEc-UA; it’s tautomer is a second candidate for the global minimum, when considering solvent effects. Despite the different photo-behavior of UA and Me-UA, there is no marked difference between their calculated conformational distributions when UA is treated in its neutral form. In contrast to neutral UA and Me-UA, zwitterionic UA exist as mixture of two non-planar conformers with strongest preference to tE geometry. Planar zwitterionic conformers represent saddle points on the ground state.
The electronic states ordering calculated by use according to the configuration interaction singles theory (CIS) was found to depend on the planarity and protonation state of the UA. Planar structures have "dark" singlet states below their strongly allowed "bright" singlet states, whereas in the non-planar structures the allowed singlet state is of lowest energy and only triplet states are lower in energy. This observation along with a comparison of UA and Me-UA spectral characteristics and photobehaviors lead us to the concept of the wavelength-dependent probability of excitation of planar and non-planar structures, causing the wavelength-dependence of trans- urocanic acid´s E/Z isomerization quantum yields.
Acknowledgement:
Financial support from Österreichische
Nationalbank (Jubiläumsfondsprojekt Nr.:7395/1 to W.R.) is gratefully
acknowledged.
References
1. (a) T. Mohammad, H. Morrison, H. HogenEsch, Urocanic acid photochemistry and photobiology. Photochem. Photobiol. 69 (1999) 115-136; (b) D.H.Hug, J.K. Hunter, D.D. Dunkerson, The potential role for urocanic acid and sunlight in the immune suppression associated with protein malnutrition, J.Photochem.Photobiol. B: Biol. 44 (1998) 117-123.
2. (a) A.M.Kammeyer, S.Pavel, S.S. Asghar, J.D. Bos, M.B.M. Teunissen, Prolonged increase of cis-urocanic acid levels in human skin and urine after single total-body ultraviolet exposures, Photochem.Photobiol. 65 (1997) 593-598; (b) M.Kinuta, K. Yao, N. Masuoka, J. Ohta, T. Teraoka, T. Ubuka, Isolation and characterization of 3-[(carboxymethyl)thio]-3-(1h-imidazol-4-yl)propanoic acid from human urine and preparation of its proposed precursor, s-[2-carboxy-1-(1h-imidazol-4-yl)ethyl]cysteine, Biochem. J. 275 (1991) 617-621.
3. (a) E.C.DeFabo, F.P. Noonan, Mechanism of immune suppression by ultraviolet irradiation in vivo. I. Evidence for the existence of a unique photoreceptor in skin and its role in photoimmunology, J.Exp.Med. 158 (1983) 84-98; (b) L.J. Webber, E.Wang, E.C.DeFabo, The effects of UVA-I (340-400 nm), UVA-II (320-340 nm) and UVA-I+II on the photoisomerization of urocanic acid in vivo, Photochem.Photobiol. 66 (1997) 484-492.
4. (a) O. Dmitrenko, W. Reischl, Previtamin D photosynthesis in vitro - review, http://www.photobiology.com/reviews/previtamin/index.html; O. Dmitrenko, W. Reischl, J. T. Vivian, J.H. Frederick, Ground and excited properties of the previtamin D conformers - contributed paper in Proceedings of the First Internet Conference on Photochemistry and Photobiology
Nov 17 -Dec 19 1997 http://www.photobiology.com/v1/dmitrenko/dmitrenko.htm;
O. Dmitrenko, J.H. Frederick, W. Reischl, Previtamin D conformations and estimation on their role in the wavelength dependence of previtamin D photosynthesis in vitro - poster contribution to Second Internet Conference on Photochemistry and Photobiology.
July 16-Sept 7 1999, http://www.photobiology.com/photobiology99/contrib/olga/index.htm;
(b) W. G. Dauben, P. E. Share, R. R. Ollmann Jr, Triene Photophysics and Photochemistry: Previtamin D3, J. Am. Chem. Soc. 110 (1988) 2548-2554; W.G. Dauben, B. Disanayaka, D.J.H. Funhoff, B.E. Kohler, D.E. Schilke, B. Zhou, Polyene 2Ag and 1Bu States and the Photochemistry of Previtamin D, J. Am. Chem. Soc. 113 (1991) 8367-8374; W. Fuss, S. Lochbrunner, The wavelength dependence of the photochemistry of previtamin D, J. Photochem. Photobiol., A.: 105 (1997) 159-164.
5. (a) H.Morrison, C.Bernasconi, G. Pandey, A wavelength effect on urocanic acid E/Z photoisomerization, Photochem.Photobiol. 40 (1984) 549-550; (b) H.Morrison, D.Avnir, C.Bernasconi, G. Fagan, Z/E photoisomerization of urocanic acid, Photochem.Photobiol. 32 (1980) 711-714; (c) K.M. Hanson, B. Li, J.D. Simon, A spectroscopic study of the epidermal ultraviolet chromophore trans-urocanic acid, J.Am.Chem. Soc. 119 (1997) 2715-2721; (b) B. Li, K.M. Hanson, J.D. Simon, Primary process of the electronic excited states of trans-urocanic acid, J.Phys. Chem.A, 101 (1997) 969-972; (d) K.M. Hanson, J.D. Simon, The origin of the wavelength-dependent photoreactivity of trans-urocanic acid. Photochem.Photobiol. 67 (1998) 538-540.
6. (a) F.D.Lewis, B.A. Yoon, The influence of intramolecular hydrogen bonding on the structure and E<->Z photoisomerization of urocanic derivatives, Res.Chem.Intermed. 21 (1995) 749-763;
(b) F.D. Lewis, D.K. Howard, J.D. Oxman, A.L. Upthagrove, S.L. Quillen, Lewis acid catalysis of photochemical reactions. Selective isomerization of b-furylacrylic and urocanic esters, J.Am. Chem. Soc. 108 (1986) 5964-5968; (c) S. Franceschi, J. Sirieix, N. Lauth-de Viguerie, M. Riviere, A. Lattes, Isomerization reactions of urocanic acid and their derivatives: comparison Z and E isomer stabilities (in French), C.R. Acad.Sci.Paris, t.2, Serie 11 c (1999) 299-303.
7. M.K. Shukla, P.C. Mishra, Electronic spectra, structure and photoisomerization of urocanic acid, Spectrochim.Acta. 51A(5) (1995) 831-838.
8. D.H.Hug, J.K.Hunter, Photochem.Photobiol., Adventitious interconversion of cis-urocanic and trans-urocanic acid by laboratory light, 59 (1994) 303-308.
9. (a) A. Lahti, M.Hotokka, K. Neuvonen, P.Ayras, A theoretical study of the conformers of trans- and cis-urocanic acid, J.Mol.Struc. (Theochem) 331 (1995) 169-179; (b) A. Lahti, M.Hotokka, K. Neuvonen, P.Ayras, Quantum-chemical gas-phase calculations on the protonation forms of trans- and cis-urocanic acid, Structural Chemistry, 8(5) (1997) 331-342; (c) A. Lahti, M.Hotokka, K. Neuvonen, G. Karlstrom, A CASSCF study on the lowest pi ->pi* excitation of urocanic acid, Int.J.Quant.Chem., 72(1) (1999) 25-37; (d) C.S. Page, M. Merchan, L. Serrano-Andres, M. Olivucci, A theoretical study of the low-lying excited states of trans- and cis- urocanic acid, J.Phys.Chem. A. 103 (1999) 9864-9871; (e) A. Lahti, M.Hotokka, K. Neuvonen, G. Karlstrom, Quantum chemical calculations on the intramolecular hydrogen bond of cis-urocanic acid, J. Mol. Struc. (Theochem) 452 (1998) 185-202.
10. (a) J.K.Laihia, H. Lemmetyinen, P. Pasanen, C.T.Jansen, Establishment of a kinetic model for urocanic acid photoisomerization, J.Photochem.Photobiol.B:Biol. 33 (1996) 211-217,
11. (a) Theoretical calculations were carried out by using the Gaussian 98 program system (ref 11b,c) with gradient geometry optimization. (b) Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Zakrzewski, V. G.; Montgomery, J. A.; Stratmann, R. E.; Burant, J. C.; Dapprich, S.; Millam, J. M.; Daniels, A. D.; Kudin, K. N.; Strain, M. C.; Farkas, O.; Tomasi, J.; Barone,V.; Cossi, M.; Cammi,R.; Mennucci, B.; Pomelli, C.; Adamo, C.; Clifford, S.; Ochterski, J.; Petersson, G. A.; Ayala, P. Y.; Cui, Q.; Morokuma, K.; Malick, D. K.; Rabuck, A. D.; Raghavachari, K.; Foresman, J. B.; Cioslowski, J.; Ortiz, J. V.; Baboul, A. G.; Stefanov, B. B.; Liu, G.; Liashenko, A.; Piskorz, P.; Komaromi, I.; Gomperts, R.; Martin, R. L.; Fox, D. J.; Keith, T.; Al-Laham, M. A.; Peng, C. Y.; Nanayakkara, A.; Gonzalez, C.; Challacombe, M.; Gill, P. M. W; Johnson, B.; Chen, W.; Wong, M. W.; Andres, J. L.; Gonzalez, C.; Head-Gordon, M.; Replogle, E. S.; Pople, J. A. Gaussian 98, Revision A.7, Gaussian, Inc., Pittsburgh PA, 1998 (c) C. Gonzlez, H.B. Schlegel, J. Chem. Phys. 1989, 90, 2154-2161. (d) C. Gonzlez, H.B. Schlegel, J. Phys. Chem. 1990, 94, 5523-5327. (e) NBO Version 3.1, Glendening, E.D.; Reed, A.E.; Carpenter, J.E.; Weinhold, F.
12. (a) A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic-behavior, Phys. Rev. A 38 (1988) 3098-3100. (b) C. Lee, W. Yang, R.G. Parr, Development of the colle-salvetti correlation-energy formula into a functional of the electron-density, Phys. Rev. B41 (1988) 785-789.
13. (a) A.D. Becke, Density-functional thermochemistry .3. The role of exact exchange , J. Chem. Phys. 98 (1993) 5648-5652. (b) P. J.Stephens, F. J. Devlin, C. F. Chabalowski, M. J. Frisch, Ab-initio calculation of vibrational absorption and circular-dichroism spectra using density-functional force-fields, J. Phys. Chem. 98 (1994) 11623-11627.
14. J.B, Foresman, T. A. Keith, K.B. Wiberg, J. Snoonian, M. J. Frisch, Solvent effects .5. Influence of cavity shape, truncation of electrostatics, and electron correlation ab initio reaction field calculations, J. Phys. Chem., 100 (1996) 16098-16104.
15. J. B. Foresman, M. Head-Gordon, J. A. Pople and M. J. Frisch, Toward a systematic molecular-orbital theory for excited-states, J. Phys. Chem. 96 (1992) 135-149.
16. O. Dmitrenko and W.Reischl, unpublished results.