| Site home page | Conference home page | Discussion |
Hydroperoxide of the furocoumarin derivative alloimperatorin as photo-Phenton agent: Inhibition of respiratory burst of neutrophils
Maria A. Chadouraa, Alla A. Kyagovaa, Natalia A. Larinaa, Alexander Ya. Potapenkoa, Waldemar Adamb, Chantu R. Saha-Möllerb
a Department of Medical and Biological Physics, Russian State Medical University, Moscow 117869, Russia, e-mail address: potap@hotmail.com.
b Institute of Organic Chemistry, University of Würzburg, 97074 Würzburg, Germany
Furocoumarins (FC) in combination with UVA radiation are used for the treatment of numerous autoimmune and skin diseases[1] involving inflammation[2]. Usually the photosensitizing effect of furocoumarins is accounted for by the ability of intercalating between DNA bases and covalent binding to pyrimidine bases under UVA irradiation[3]. However, other mechanisms of furocoumarin action are possible. Some FC, such as imperatorin and alloimperatorin (Al), form hydroperoxides by photooxidation. Hydroperoxides of these FC readily intercalate between DNA bases. UVA irradiation of furocoumarin hydroperoxides generates hydroxyl radicals, which oxidize DNA efficiently. That is, FC hydrperoxides function as photo-Phenton reagents: they are the source of hydroxyl radicals generated in close vicinity of DNA[4]. These reactions result in mutagenic and antiproliferative effects on the culture of mouse lymphoma cells[5]. Besides, the intraperitoneal injection of alloimperatorin hydroperoxide (AlOOH)[6] and the photooxidation products of other FC[7] to mice were shown to inhibit immune reactions involving inflammation: The delayed type hypersensitivity and contact hypersensitivity reactions. In these reactions AlOOH did not function as photo-Phenton reagent, because the mice and hence AlOOH were not subjected to UVA irradiation, these were dark effects.
 |
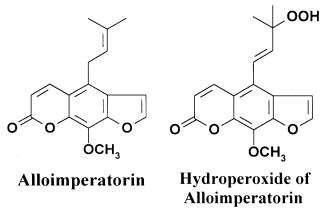
Fig.1. The structures of alloimperatorin and alloimperatorin hydroperoxide.
In the present paper dark and photosensitized effects of Al and its hydroperoxide (Fig. 1) on the functional activity of neutrophils were studied. The functional activity of neutrophils was estimated by respiratory burst (RB) registered by luminol-dependent chemiluminescence (ChL). Neutrophils were stimulated either by opsonized zymosan A, phagocytosis activator[8], or by phorbol-12-myristate-13-acetate (PMA), the activator of proteinkinase C (PKC)[9].
The reagents, alloimperatorin and alloimperatorin hydroxide were synthesized as described elsewhere[10]; D-mannitol, Sigmacote silicon, phorbol-12-myristate-13-acetate (PMA), zymosan A, peptone, and luminol were purchased from “Sigma” (USA); the phosphate buffered saline (PBS) was of “ICN” (USA) production; acetic acid and ethanol were of “Reakhim” (Russia); Hanks solution was obtained from the Institute of Poliomyelytis, Moscow, Russia. Distilled ethanol and bidistilled water were used for the solutions.
UVA irradiation
Neutrophils suspension containing furocoumarins was irradiated with the light of a “Narva 125-2” lamp (Germany) with emission maximum at l=365 nm. Light intensity (at l=365 nm) measured with a calibrated photocell “Waldman UV meter” was 15 W/m2.
All suspensions and solutions were stirred with a magnetic stirrer during UVA irradiation.
Isolation of neutrophils[11]
Rats (14-15 weeks old) were injected intraperitoneally with 2 ml of 2% peptone solution in PBS. Twenty ml of Hanks solution (37oC, pH 7.4) were injected in the abdominal cavity 19-20 h later. After 2 to 3 min massaging, abdominal cavity was opened and exudate was taken by using a Pasteur pipette. The peritoneal exudate was filtered through two layers of nylon material and placed in siliconized glass tubes. Cells were washed twice in Hanks solution by centrifugation at 400 g for 10 min. The pellet was resuspended in 1 ml of Hanks solution. The amount of cells in the suspension was counted in microscope hemocytometer by using 3% acetic acid to remove erythrocytes. Cell suspension was diluted with Hanks solution up to the concentration 20·106 cells/ml.
With this technique of cell isolation, about 90% neutrophils in the peritoneal exudate was achieved. Morphology of cells in an assay was determined by staining procedure by Romanovsky-Gimsa[12].
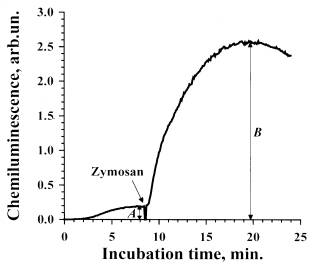 |
Fig.2. A typical curve of zymosan-stimulated luminol-dependent chemiluminescence of rat peritoneal neutrophils.
To an aliquot of the suspension of furocoumarin-treated cells 50 ml of luminol solution were added. The sample was incubated for 8-9 min at 37oC in the cuvette of a chemoluminometer (CLM-3 ADInstuments). Spontaneous ChL of the cells was registered during the incubation. Then, the solution of the stimulant (zymosan in this case) of the respiratory burst was added. Subsequently, ChL response of the cells to the stimulant was recorded for 15-20 min. The amplitude of stimulated ChL was estimated according to the formula C=(B-A), where A is the intensity of spontaneous ChL, B is intensity of stimulated ChL in the maximum.
Opsonized zymosan preparation
Zymosan A was opsonized with rat blood serum[13]. For this purpose, 25 mg of zymosan A were mixed with 1.25 ml of Hanks solution and 1.25 ml of blood serum, and incubated at 37oC. During incubation, the suspension was stirred every 8-10 min. Then, zymosan was 3 times washed in Hanks solution by centrifuging at 400 g for 10 min. The opsonized zymosan was resuspended in 2.5 ml of Hanks solution, divided into aliquots, and stored at -20oC.
Photosensitizing effects of Al and AlOOH.
110 ml of Al or AlOOH solution (10-5 M, in PBS with 1% of ethanol ) were added in a glass siliconized vessel containing 720 ml of Hanks solution and 120 ml of cell suspension (2·106 cells/ml). In the control samples, 110 ml of FC solution were replaced by 110 ml PBS with 1% of ethanol. The samples were placed on ice and irradiated (I=15 W/m2) at vigorous stirring with a magnetic stirrer. Then, 890 ml of the irradiated suspension were placed in the cuvette of chemiluminometer, 50 ml of luminol (5·10-4 M) were added, and spontaneous chemiluminescence (ChL) of the cells was registered at 37oC. After spontaneous ChL reached plateau (in 8-9 min), 50 ml of either zymosan A (0.5 mg/ml) or PMA (10 ng/ml) were added. A typical ChL curve and the estimation of its amplitude are shown in Fig. 2 (for details See Figure Legend).
Dark effects of AlOOH.
Dark effects of AlOOH on ChL of cells was investigated as follows. The samples were prepared: 90 ml of AlOOH solution (in PBS with 1% of ethanol) in varied concentration were added to 800 ml of cell suspension (2.5·106 cells/ml). The samples were incubated on ice for 5 min, placed in the cuvette of a chemiluminometer, 50 ml of luminol solution (5·10-4 M) were added, and ChL was registered at 37oC, as described above.
The combined action of Al and UVA irradiation on the respiratory burst (RB) of neutrophils stimulated by zymosan was investigated at the first stage of our study.
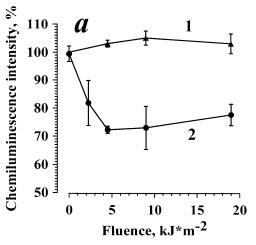
In Fig. 3a data are presented on the dependence of zymosan-stimulated ChL on the dose of irradiation in the presence of Al (curve 2). For the control, zymosan-stimulated ChL of cells irradiated without Al was measured (curve 1). The amplitude of zymosan-stimulated ChL of cells incubated in PBS in the dark with 0.1% of ethanol was arbitrarily set as 100%. It was seen that in the dark Al produced no effect on RB of neutrophils. In the range 0.9-4.5 kJ/m2 the combined action of Al and UVA irradiation (curve 2) induced a slight (not greater than 15 %) dose-dependent inhibition of ChL amplitude. At the doses higher than 4.5 kJ/m2, no inhibitory effect was observed, and at 9 kJ/m2 the effects of irradiation with and without Al were equal, i.e. curve 2 reached curve 1. UVA irradiation of cells without Al (curve 1) exerted practically no effect on the respiratory burst.
It is known that in Al photooxidation, a number of photoproducts are formed, AlOOH being one of these products[10]. To test the hypothesis that the observed Al effects are due to AlOOH formation, we have investigated the ability of AlOOH to modulate ChL response to zymosan of neutrophils in darkness. For this purpose, neutrophils were incubated in the dark at 4oC for 5 min in the presence of AlOOH in varied concentrations (from 10-10 M to 10-6 M), then, cellular ChL was registered at 37oC. Dark effect of AlOOH on zymosan-stimulated neutrophil ChL for the studied range of concentrations was absent (data are not presented). Thus, the effects photosensitized by alloimperatorin could not be conditioned solely by AlOOH formation and its subsequent dark action
 |
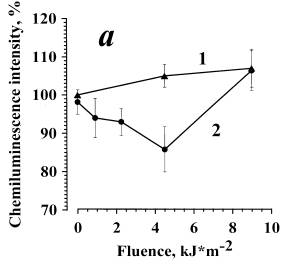 |
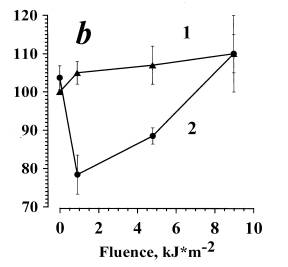
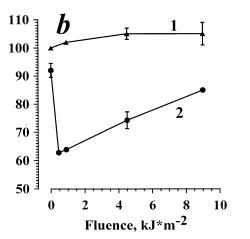
Fig. 3. The inhibition of the opsonized zymosan-stimulated respiratory burst of neutrophils photosensitized by alloimperatorin (a) and alloimperatorin hydroperoxide (b).
(a) is combined action of Al and UVA irradiation; (b) is combined action of AlOOH and UVA irradiation. Curves 1 are for: cells + PBS + 0.1% of ethanol + UVA. Curves 2 are for: cells + Al or AlOOH (10-6 M) +UVA.
The mean values of 5 independent experiments±SEM are presented.
Alloimperatorin hydroperoxide is known to be chromophore, under UVA light it decomposes to generate of hydroxyl radicals[4]. Therefore, we investigated AlOOH ability to photosensitize the modifications of RB.
In Fig. 3b, data are presented on the dependence of the amplitude of zymosan-stimulated cellular ChL on the dose of irradiation in the presence of AlOOH (10-6 M). At low irradiation doses (0.9 kJ/m2), a considerable ChL inhibition (curve 2) was observed. The effect is eliminated with an increase in the irradiation dose. In the control samples, ChL of the cells irradiated without AlOOH was almost unchanged (curve 1), like in the experiment shown by curve 1 (Fig. 3a). Thus, AlOOH photosensitized inhibition of zymosan-stimulated ChL of neutrophils was confirmed.
The hydroxyl radicals formed in AlOOH photolysis are known to play the main role in the mutagenous and antiproliferative effects on mouse lymphoma cells[14]. To estimate the contribution of hydroxyl radicals in the inhibition of neutrophils RB, we studied the effects of hydroxyl radical trap, mannitol. The cells were irradiated with UVA light at the doses of 0.9 and 4.8 kJ/m2 in the presence of AlOOH (10-6 M) and mannitol (10-5 M) (Fig. 4). In the control samples, cells were irradiated in the presence of AlOOH but without mannitol .Mannitol in the concentration used in our experiments (10-5 M) did not influence the ChL amplitude of non-irradiated cells both in the presence (Fig. 4) and in the absence of AlOOH (data are not presented).
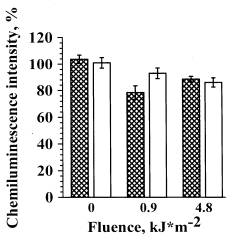 |
Shaded bars are the samples containing 10-5 M mannitol, empty bars are the samples without mannitol.
At the irradiation dose of 0.9 kJ/m2, inducing the maximal effect of ChL inhibition (Fig. 2b, curve 2) mannitol led to significant elimination of the effect photosensitized by hydroperoxide. At a higher irradiation dose (4.8 kJ/m2) mannitol did not produce such effect. Thus, it may be concluded that RB inhibition, photosensitized by AlOOH at low irradiation doses (0.9 kJ/m2) involves hydroxyl radicals, while at higher doses other reactive species are involved.
The data shown in Figs. 3 and 4 allow to suggest that Al-photosensitized inhibition of ChL response of neutrophils may proceed through photogeneration and subsequent photolysis of AlOOH as shown in the following scheme:
![]() inhibition of zymosan-stimulated
ChL.
inhibition of zymosan-stimulated
ChL.
This suggestion is supported by the following experimental data: the shape of the UVA-dose dependences for AlOOH (Fig. 3b, curve 2) and for Al (Fig. 3a, curve 2) are similar. Thus, at low irradiation doses, the inhibition of ChL-response was observed and its elimination at higher doses. The effect photosensitized by AlOOH was shown to be shifted towards the lower irradiation doses (Fig. 3b, curve 2) as compared to Al (Fig. 3a, curve 2). The shift of the dose dependence in the presence of Al (Fig. 3a, curve 2) towards the higher irradiation doses may be conditioned by the fact that AlOOH must first be formed from Al, and subsequently AlOOH can photosensitize damage of neutrophils.
It is known that stimulation of phagocyting cells by zymosan proceeds through the opsonine receptor, whose activation leads to signal transduction to the cell by two independent pathways. One of them is initiated by the activation of phospholipase C and involves the activation of proteinkinase C (PKC), the other is started by phosphoinositol-3-kinases which initiate the phosphorylation of various cytoplasmic kinases[9;15]. Thus, we investigated the ability of Al and AlOOH to influence the production of reactive oxygen species by neutrophils via the activation of proteinkinase C. As a stimulant, PMA was chosen which is known as the direct activator of PKC[9].
 |
 |
Curves 1 are for: cells + PBS + 0.1% of ethanol + UVA. Curves 2 are for: cells + Al or AlOOH (10-6 M) + UVA.
(a) mean values of 5 independent experiments±SEM are presented.
(b) In the curve 2, the points for the irradiation doses 0 and 4.5 kJ/m2 were obtained as the mean of 3 independent experiments±SEM; the points for the irradiation doses of 0.45, 0.9, and 9 kJ/m2 were obtained from a single experiment at each dose.
The addition of AlOOH (10-6 M) to the suspension without irradiation resulted in a slight (about 10%) inhibition of the respiratory burst (Fig. 5b, curve 2). Cell irradiation in the presence of AlOOH led to the inhibition of PMA-stimulated ChL (Fig. 5b, curve 2). This particular experiment (curve 2) did not receive sufficient statistical treatment, thus, we shall not discuss the shape of the dose curve.
The obtained experimental data show, that Al- or AlOOH-photosensitized inhibition of ChL of neutrophils is more pronounced when cells were stimulated with PMA (Fig. 5a, b, curves 2) as compared to zymosan-stimulated cells (Fig. 3a,b, curves 2). Moreover, the inhibition of the response to PMA was not eliminated with the increase in the irradiation dose (Fig. 5a, curve 2). It is known that opsonized zymosan initiates several pathways of cell activation, while PMA is a specific activator of only one of these pathways (involving PKC)[9;15]. Both, Al and AlOOH photosensitize inhibition of proteinkinase C involving pathway (Fig. 5). Thus weaker inhibiting effect in the case of zymosan-stimulated cells may be explained as follows: when the proteinkinase C (PKC) pathway is blocked, the other parallel, shunting pathway is not blocked, and cells may be activated by this different unblocked pathway.
Our data strongly indicate that Al and AlOOH photosensitize inhibition of PKC-mediated mechanism of reactive oxygen species production. Earlier it was shown that FC hydroperoxides function as intercalating photo-Fenton reagents for oxidative DNA modification by hydroxyl radicals[4]. The results of the present paper suggest that not only DNA may be the molecular target of AlOOH-photosensitized reactions. AlOOH as photo-Phenton reagent efficiently damages the biochemical PKC-mediated system of neutrophils.
ACKNOWLEDGEMENT
1 D. Bethea, B. Fullmer, S. Syed, G. Seltzer, J. Tiano, C. Rischko, L. Gillespie, D. Brown and F.P. Gasparro, Psoralen photobiology and photochemotherapy: 50 years of science and medicine. J Dermatol Sci, 19:2 (1999) 78-88.
2 B.P. Peters, F.G. Weissman and M.A. Gill, Pathophysiology and treatment of psoriasis. Am J Health Syst Pharm, 57, No. 7 (2000) 645-659.
3 F. Dall'Acqua, S.M. Magno, F. Zambon and G. Rodighiero, Kinetic analysis of the photoreaction (365 nm) between psoralen and DNA. Photochem.Photobiol., 29, No. 3 (1979) 489-495.
4 W. Adam, M. Berger, J. Cadet, F. Dall'Acqua, B. Epe, S. Frank, D. Ramaiah, S. Raoul, C.R. Saha-Möller and D. Vedaldi, Photochemistry and photobiology of furocoumarin hydroperoxides derived from imperatorin: novel intercalating photo-Fenton reagents for oxidative DNA modification by hydroxyl radicals. Photochem.Photobiol., 63 (1996) 768-778.
5 B. Epe, M. Haring, D. Ramaiah, H. Stopper, M.M. Abou-Elzahab, W. Adam and C.R. Saha-Möller, DNA damage induced by furocoumarin hydroperoxides plus UV (360 nm). Carcinogenesis, 14, No.11 (1993) 2271-2276.
6 E.S. Andina, A.A. Kyagova, N.A. Yurikova, M.B. Neklyukova, A.Y. Potapenko, W. Adam and C.R. Saha-Möller, Immunosuppression induced by the photooxidation products of furocoumarins (psoralens). In M.F. Holick and E.G. Jung (eds.), Biologic Effects of Light, Kluger Academic Publishers, Boston/London/Dordrecht, 1999, pp. 213-216.
7 A.A. Kyagova, N.N. Zhuravel, M.M. Malakhov, E.P. Lysenko, W. Adam, C.R. Saha-Möller and A.Ya. Potapenko, Suppression of delayed-type hypersensitivity and hemolysis induced by previously photooxidized psoralen: effect of fluence rate and psoralen concentration. Photochem.Photobiol., 65, No.4 (1997) 694-700.
8 Y.Y. Maeda, J. Hamuro, Y.O. Yamada, K. Ishimura and G. Chihara, The nature of immunopotentiation by the anti-tumour polysaccharide lentinan and the significance of biogenic amines in its action. In Immunopotentiation. Ciba Foundation Symposium 18 (new series)., Associated Scientific Publishers, Amsterdam, 1973, pp. 259-281.
9 A. Karlsson, J.B. Nixon and L.C. McPhail, Phorbol myristate acetate induces neutrophil NADPH-oxidase activity by two separate signal transduction pathways: dependent or independent of phosphatidylinositol 3-kinase. J Leukoc Biol, 67 (2000) 396-404.
10 M. Abou-Elzahab, W. Adam and C.R. Saha-Möller, Photooxidation of some potentially skin-photosensitizing furocoumarins: Imperatorin, alloimperatorin and its methyl ether and acetate derivatives. Liebigs Ann.Chem., (1991) 967-970.
11 P. Dörfling and S. Wischner, Isolation of macrophages from suspension of splenicytes. In H. Friemel (ed.), Immunologische Arbeitsmethoden, VEB Gustav Fischer Verlag, Jena, 1984,
12 L.I. Roskin and L.V. Levinson, Microscopic technique., Sovetskaya Nauka, Moscow, 1957, pp. 59-61.
13 D. Marcus-Bagley and C.A. Alper, Methods for allotyping complement proteins. In N.R. Rose, E.C. de Macario, J.L. Fahey, H. Friedman and G.M. Penn (eds.), Manual of Clinical Laboratory Immunology., American Society for Microbiology, Washington, D.C., 1992, pp. 124-141.
14 M. Möller, H. Stopper, M. Haring, Y. Schleger, B. Epe, W. Adam and C.R. Saha-Möller, Genotoxicity induced by furocoumarin hydroperoxides in mammalian cells upon UVA irradiation. Biochem Biophys Res Commun, 216 (1995) 693-701.
15 C. Vaissiere, V. Le Cabec and I. Maridonneau-Parini, NADPH oxidase is functionally assembled in specific granules during activation of human neutrophils. J Leukoc Biol, 65, No. 5 (1999) 629-634.