
In Vivo Documentation
of Photochemical Internalization: a Novel Approach to Site Specific Cancer Therapy
Pål K. Selbo1,
Gowsala Sivam2,4, Øystein Fodstad2, Kirsten Sandvig3
and Kristian Berg1
1Department of Biophysics, 2Department of Tumor Biology,
3Department of Biochemistry, Institute for Cancer Research,
The Norwegian Radium Hospital, Montebello, 0310 Oslo, Norway.
4Bastyr University Research Institute, Kenmore, WA, USA
ABSTRACT Photochemical
internalization (PCI) is a novel technology for site-specific delivery of several
types of membrane impermeable molecules to the cytosol of target cells1-5.
Our technology is based on photochemical induced release of endocytosed macromolecules
from endosomes and lysosomes into the cytosol (Fig. 1). The purpose of this study
was to evaluate the therapeutic potential of photochemical internalization of
the type I ribosomal inactivating protein gelonin in an animal model. In this
report we present in vivo documentation for photochemical internalization
of the type I ribosome-inactivating protein gelonin. Complete remission in 67%
of the treated mice were induced.
INTRODUCTION
- Several photosensitizers, including AlPcS2a
(aluminum phthalocyanine with 2 sulfonate groups on adjacent rings) used in
the present study, localize in the membranes of endosomes and lysosomes in
vitro, and light activation of such sensitizers may destroy these membranes.
Thus, we have recently reported that this principle can be utilized for transfer
of a variety of macromolecules from these vesicles into the cytosol by PCI1-5.
- Ribosome-inactivating proteins (RIPs) are N-glycosidases,
which catalytically and irreversibly inactivate eukaryotic ribosomes. These
proteins can have extremely cytotoxic effects and only one molecule can be
sufficient to kill a cell. RIPs therefore have a great potential in treatment
of cancer. Gelonin, a glycosylated type I RIP, is internalized by either fluid
phase endocytosis or by binding to galactosyl or mannose receptors. The low
cytotoxicity of gelonin is due to inefficient uptake and penetration to the
cytosol, and the transport of the toxin to lysosomes where it is degraded.
- In the present work the in vivo potential of
this method for achieving light directed, tumor specific delivery of the type
I RIP gelonin has been explored.
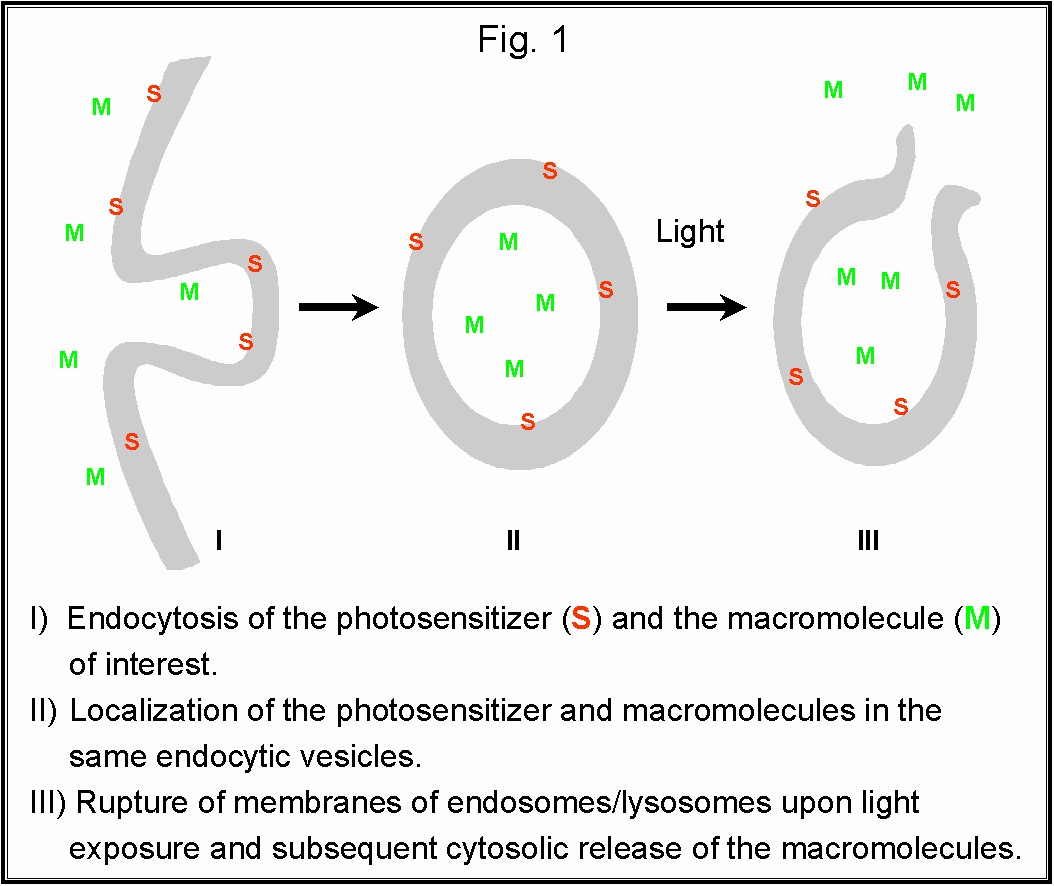
RESULTS AND DISCUSSION
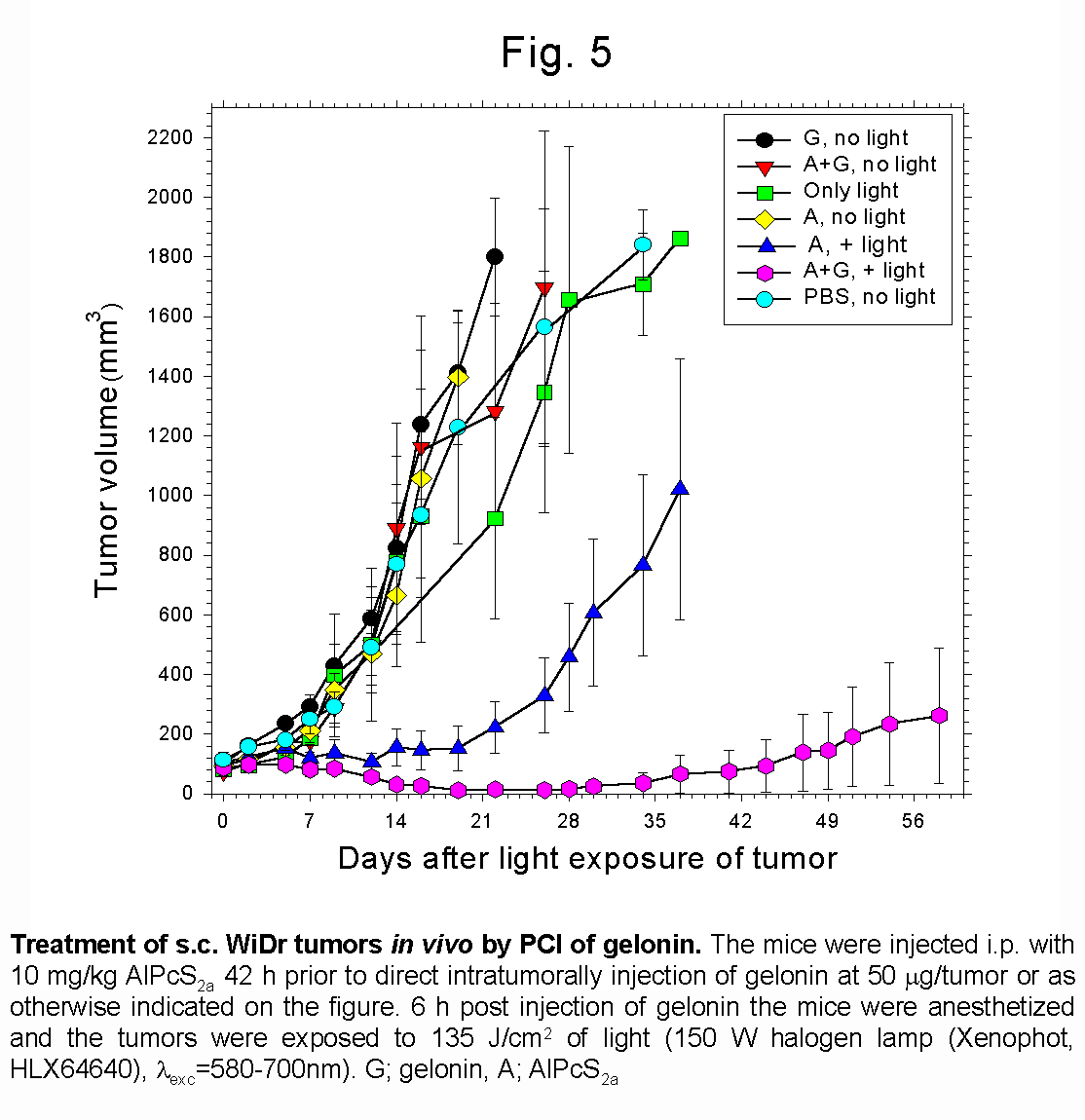
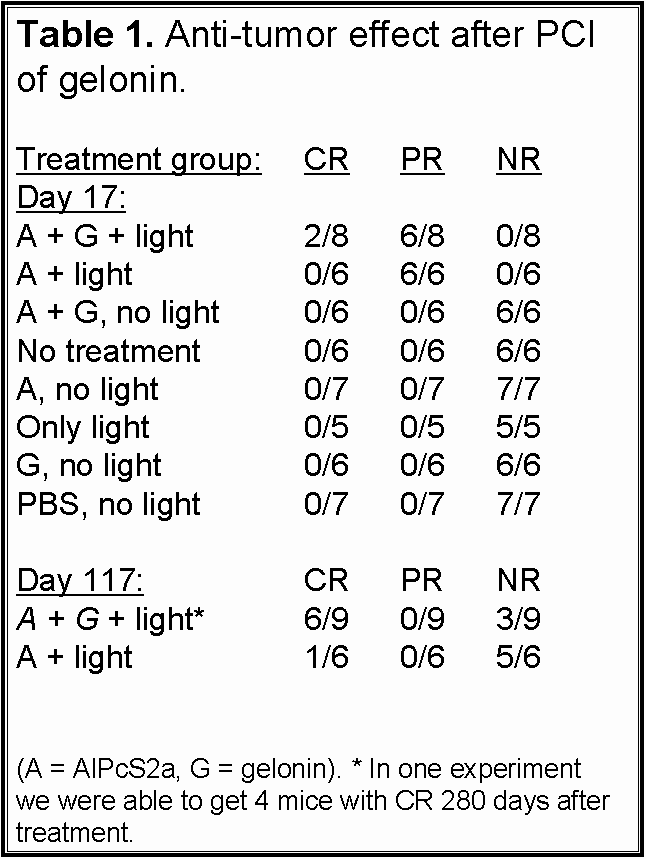
The synergistic effect on tumor growth (Fig. 5) and cure (Tab.1) obtained by combining
the ribosome-inactivating protein gelonin with photoactivation of AlPcS2a
is most likely due to PCI of gelonin and not caused by other factors. Results
supporting PCI as a cause of the synergistic effects are:
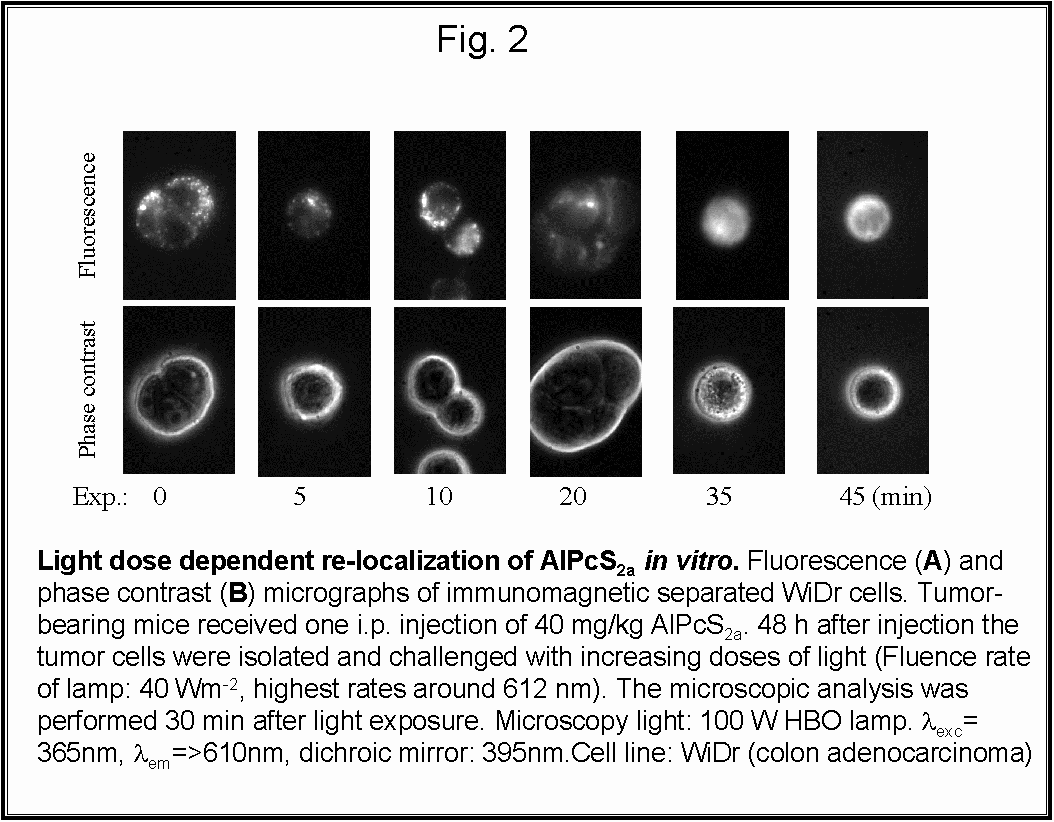
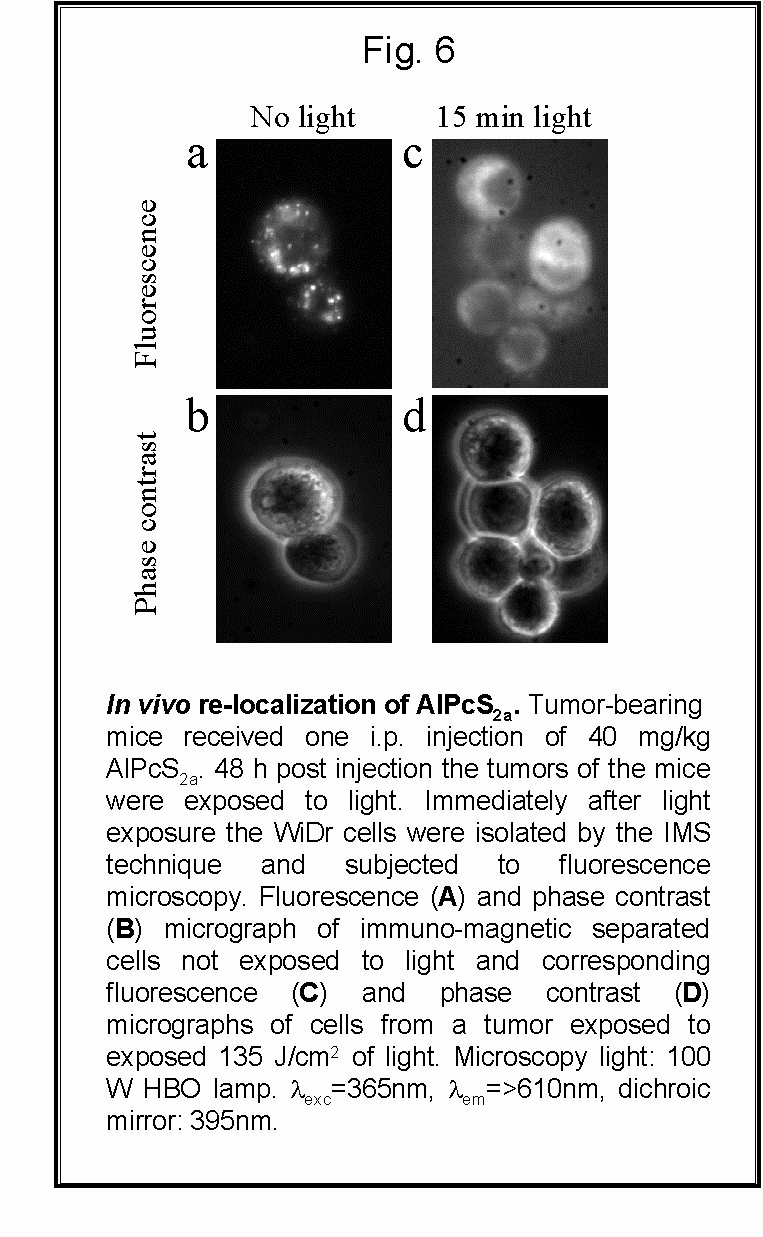
- Fig 2 and 6: AlPcS2a is located in
endocytic vesicles/lysosomes in the isolated WiDr tumor cells. The photosensitizer
can be released from its vesicle localization upon exposure to light both
in vitro and in vivo.
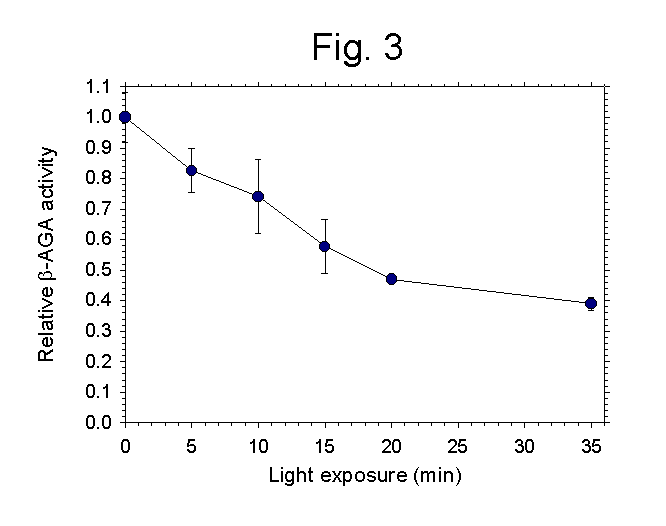
- Fig 3: The b-AGA
enzyme inactivation assay is the proof of a lysosomal localization of the
photosensitizer.
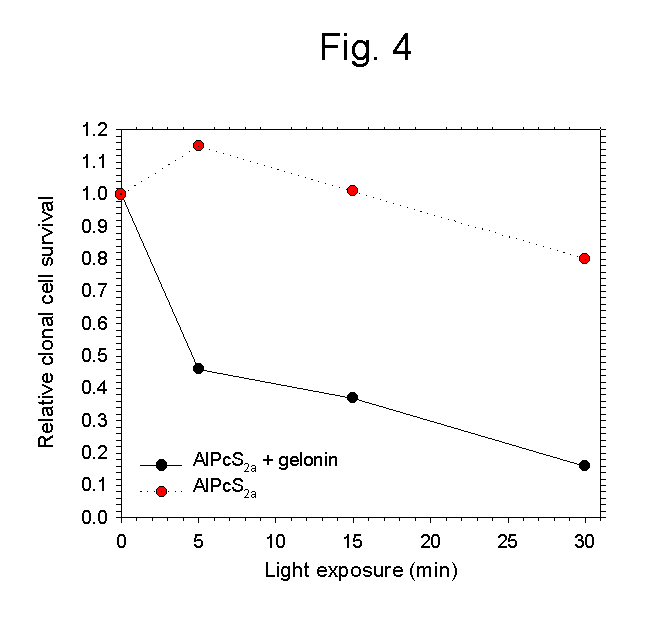
- Fig 4: Gelonin added to isolated WiDr cells, pre-treated
in vivo with AlPcS2a, became cytotoxic upon light exposure
in vitro.
In addition: AlPcS2a in WiDr cells in culture co-localize with fluorescence-labeled
gelonin and both compounds are released from endocytic vesicles into the cytosol
upon light treatment3. Photosensitizers not located in endocytic vesicles
have previously been shown unsuccessful in activating the cytotoxic potential
of gelonin1
CONCLUSION
- For the first time we have documented by the use of
gelonin that PCI is a unique technology for biological activation of macromolecules
in vivo by stimulating the transfer of the macromolecules into the
cytosol.
- The results demonstrate that the synergistic effect
of combining photoactivation of photosensitizer located in endocytic vesicles
and gelonin is indeed due to PCI of gelonin.
- Effective PCI of macromolecules at sub-toxic photochemical
doses opens up possibilities for treatment of diseases different from cancer.
- Our results presented here are encouraging and demonstrate
that PCI of gelonin provides a new alternative approach for the treatment
of cancer.
REFERENCES
1) Berg,K., Selbo,P.K
, Prasmickaite,L., Tjelle,T.E., Kjølsrud,S., Rodal, G.H., Anholt,H., Sandvig,K.,
Moan,J., Gaudernack,G., Fodstad,Ø., Rodal,S.K. and Høgset,A. (1999)
Photochemical internalization. A novel technology for site-specific delivery of
macromolecules into cytosol. Cancer Res., 59: 1180-1183. 2) Selbo, P.K., Sivam,G.,
Fodstad,Ø., Sandvig.K. and Berg,K. Photochemical internalisation increases
the cytotoxic effect of the immunotoxin MOC31-gelonin. (2000) Int.J.Cancer, 87:
853-859. 3) Selbo,P.K., Sandvig,K., Kirveliene,V. and Berg,K. Release of gelonin
from endosomes and lysosomes to cytosol by photochemical internalization. (2000)
Biochem.Biophys.Acta, 1475: 307-313. 4) Høgset,A., Prasmickaite,L., Tjelle,T.E.
and Berg,K (2000) Photochemical transfection, a new technology for light-induced,
site-directed gene delivery. Hum. Gene Ther., 11: 869-880. 5)
http://www.uio.no/~krberg/index.htm
(home page of the PCI-group)
ACKNOWLEDGEMENTS
This investigation was supported by grants from The
Norwegian Cancer Society (# 96138/007), The Norwegian Radium Hospital and PhotoCure
ASA. We also thank Dr. Qian Peng for helpful discussion and Fabio Apricena, Siri
Juell and Øyvind Edon Olsen for technical assistance.








