1. Introduction
Molecular oxygen is one of the most important substance on the earth. Oxygen comprises 21% of the atmosphere, 89 % of seawater by weight, and at least 47% of the Earth's crust.. Almost all living organisms utilize oxygen for energy generation and respiration. In the 1840s Michael Faraday discovered that oxygen is attracted to a magnet. Almost a century later, in 1925, Robert Mulliken explained why oxygen is magnetic using the recently developed quantum theory. His analysis shows that molecular oxygen has two unpaired electrons in its lowest energy state. The intrinsic magnetism of electrons had been proposed by Goudsmit and Uhlenbeck only the prior year. The existence of unpaired valence electrons in a stable molecule is very rare in nature and confers high chemical reactivity. Hundreds of photochemical reactions involving oxygen have been studied in the laboratory. Photolysis of Acolorless@ substances is initiated by exposure to ultraviolet radiation (UV). Alternatively, absorption of visible light by a coloured substance present in small amounts may initiate physical or chemical changes in a substrate. This process is photosensitisation. Many photosensitised processes require molecular oxygen in the initial step, e.g., dye-photosensitised oxidation of cholesterol and photodynamic therapy (PDT). Colour photography and green plant photosynthesis are examples of photosensitisation that do not require oxygen. The specific role of oxygen in photochemical processes was unresolved until the 1960s. A few early workers suggested that Aactive oxygen@ is a key intermediate but this mechanism was generally discounted. However, the importance of Aactive oxygen@ was demonstrated in 1931 by Hans Kautsky, a ADocent@ (assistant professor) at Heidelberg University, who proceeded to investigate its properties for another 25 years. The photosensitiser in Kautsky=s original experiments was the dye trypaflavine adsorbed on silica gel. Kautsky found that the presence of oxygen extinguishes or quenches the fluorescence of the trypaflavine, and in the same system, the colorless form of the dye malachite green (the leuco dye) , also adsorbed on silica gel, was converted to the normal bright green form. He correctly deduced that energy is transferred from the optically-excited trypaflavine molecule to molecular oxygen and the resultant Aactive oxygen@ is responsible for the oxidation of leuco malachite green. Kautsky=s elegant work and careful reasoning were largely ignored until 1964, when new experimental evidence confirmed the role of Aactive oxygen@ in photosensitisation and photo-oxygenation reactions. The unstable, energy-rich Aactive oxygen@ identified by Kautsky is singlet molecular oxygen or Asinglet oxygen@ (1O2). There is now a vast literature about 1O2 and many special conferences have been devoted to this subject. This article reviews the properties of 1O2, including its molecular structure, methods of generation and detection, chemical reactivity, and role in some important chemical and biological systems.
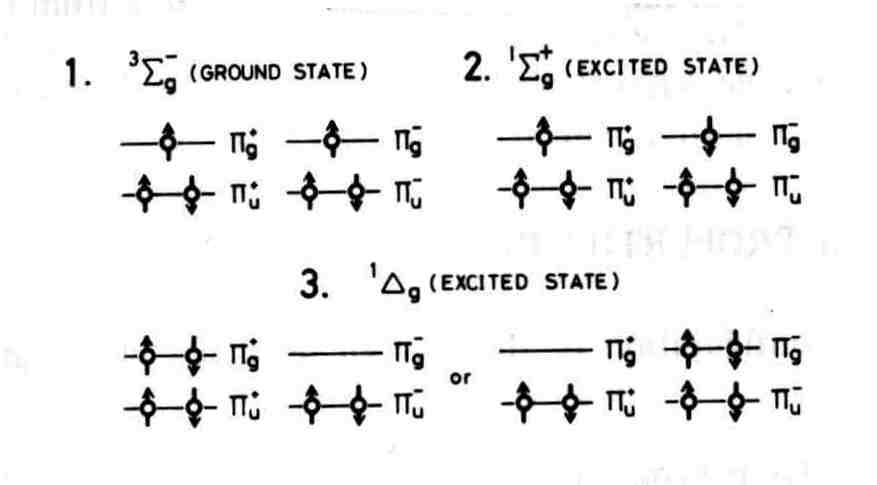
2. The Structure of Molecular Oxygen
The quantum theory of atomic and molecular structure is required to explain the unique properties of molecular oxygen. The electron configuration of atomic oxygen is: O Y (1s)89(2s)89(2px)8 (2py)8 (2pz)89. The LCAO model leads to the following occupation of molecular orbitals (MO) in the ground state: O2Y(1sg)2(1su)2(2sg)2(2su)2 (3sg)2(1pu)4(1pg)2. The electronic properties of oxygen are determined by the six electrons in p MO. The spin configuration of the lowest energy state is: O2Y (core) (px)89 (py)89 (p*x )8 (p*y)8 . The spectroscopic term for this state is 3Sg G. The unpaired electrons in two different MO account for the paramagnetism of molecular oxygen. The electron occupancy of the ground state and lower energy excited states are depicted in Figure 1. Two paired electrons occupy the same pg MOs in the excited states labeled 1Dg. O2 (1Dg+) is the lower energy species that reacts chemically as 1O2. The excitation energy is 0.98 eV (22.5 kcal/mole) and the radiative decay lifetime is 45 minutes at very low gas pressures. However, collisions with other molecules induce much shorter lifetime, e.g. 14 minutes in oxygen gas at 760 torr. The higher energy excited state is 1Sg+. In this state two paired electrons occupy two different pg MOs . The excitation energy is 1.63 eV (37.5 kcal/mole) and the decay lifetime is 7 seconds. The higher energy state O2 ( 1Sg+) is rapidly converted to O2(1Dg+) in condensed media.

Figure 1. Occupation of molecular orbitals in oxygen.
3. Production of Singlet Oxygen
1
O2 is generated by many different types of reactions. 1Dg+ is the only important state in condensed media. Both 1Sg+ and 1Dg+ are significant in the gas phase because the two states are inter-convertible via the reactions:O2(1
Dg+) + O2(1Dg+) Y O2( 1Sg+) + O2(3Sg G)O2(1
Sg+ ) + Q + M Y O2(1Dg+) + Q+ Mwhere Q is a foreign molecule and M is the third body required for conservation of energy and momentum in the gas phase.
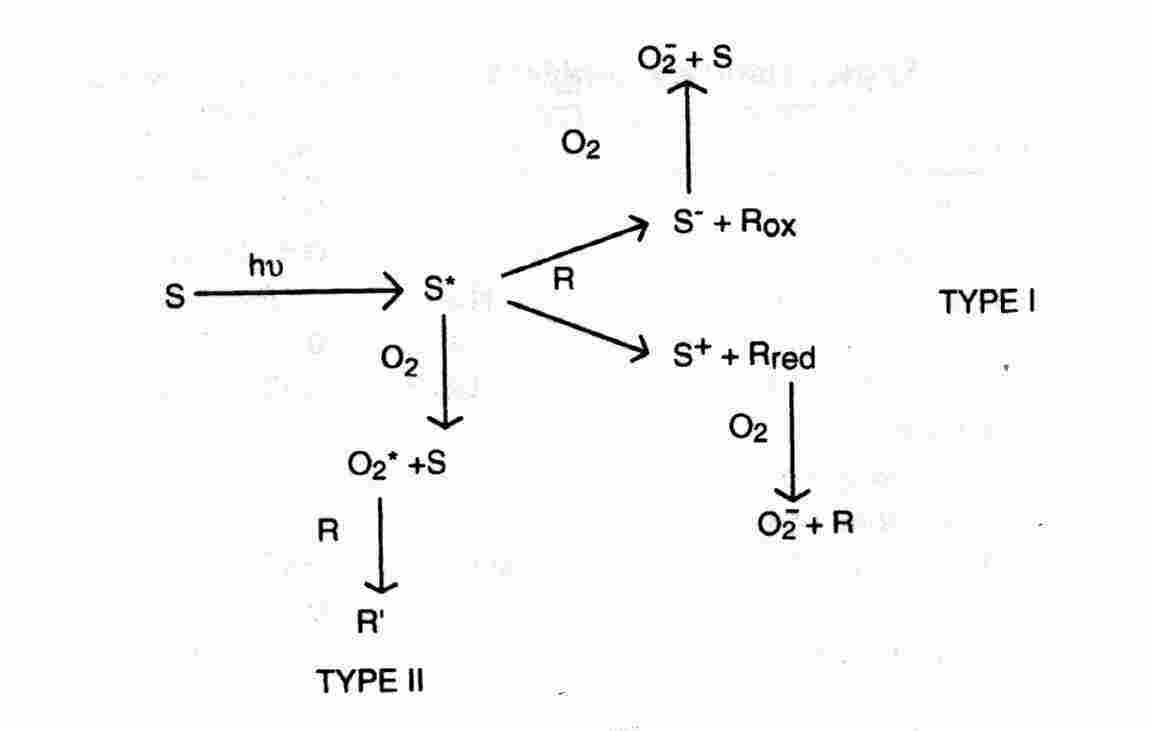
3.1. Photosensitisatio
n: Photosensitisation refers to a light-activated process that requires the presence of a light-absorbing substance, the photosensitiser, that initiate a physical, chemical, or biological process in a non-absorbing substrate. The pathway in which a photosensitiser triplet state reacts first with a substrate other than molecular oxygen is termed Type I. In the alternative Type II pathway the photosensitiser triplet state reacts first with molecular oxygen. Type II photosensitisation of a biological system is referred to as photodynamic action. (Some authors limit the designation photodynamic action to a Type II process mediated by 1O2 but this convention is not uniformly followed. ) In the experiments of Kautsky, energy transfer to gaseous oxygen from an optically-excited dye adsorbed on silica gel led to 1O2. More frequently, the photosensitiser, oxygen, and substrates are dissolved in a liquid or rigid medium. The basic reactions of a dye-like molecule are depicted in Figure 2 which is termed the Jablonsky diagram. The primary act of light absorption elevates one electron in the ground singlet state (1S0) to an excited vibronic level of an excited singlet state, 1S1, 1S2, 1S3, .., without a change of spin direction. This process requires about 10-15 seconds. The higher energy electronic levels 1S2, 1S3,... undergo a Ahorizontal@ internal conversion (IC) to a vibrationally-excited level of the 1S1 state, followed by a vibrational relaxation (vr) in which the excess vibrational energy is dissipated as heat after about 10-11 seconds. This process leaves the excited molecule in the thermally-equilibrated 1S1 state or fluorescent state. The typical lifetime of a fluorescent state is 10-9 - 10-8 seconds.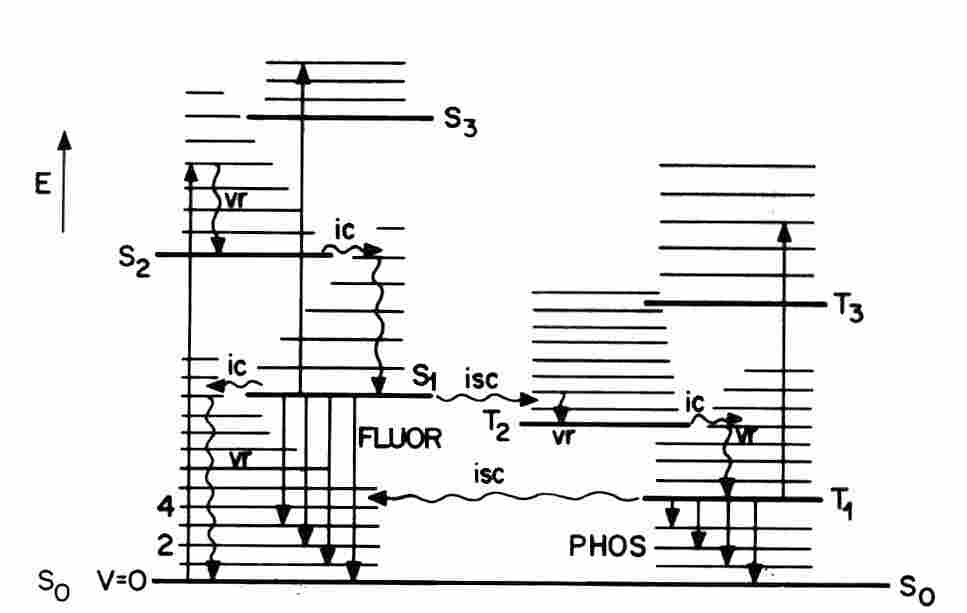
Figure 2. The Joblonski diagram for optical excitation of photosensitising molecule.
The excitation energy of an 1S1 state may be released by one of several different processes, with relative probabilities that depend on the molecular structure and the environment. The unexcited dye is restored by emission of a photon as fluorescence (FLUOR) and by radiationless energy transfer to the medium as heat. Molecular fluorescence has two characteristic properties: (A) The fluorescence emission spectrum is located on the long wavelength or Ared@ side of the absorption spectrum owing to the energy dissipated as heat prior to the population of the fluorescent state; this phenomenon is the Stokes= shift. (B) The fluorescence emission spectrum does not depend on the excitation wavelength. An alternative relaxation process is the transfer of the 1S1 excitation energy to an unexcited molecule of the same or different type. Efficient energy transfer from a donor to an acceptor must be Adownhill@. Several types of excitation energy transfer mechanisms have been identified. The Along-range@ Förster process involves a "dipole-dipole" interaction between the excited singlet states of the donor and acceptor. The transfer efficiency depends on the spectral overlap of the donor emission band and the acceptor absorption band, the inverse-sixth power of the intermolecular separation, and the relative donor-acceptor orientation. An excited acceptor may decay by emitting its characteristic fluorescence. This process is sensitised fluorescence.
Intersystem crossing (ISC) is another possible fate of a fluorescent state. ISC requires a Aspin flip@ and populates the lowest energy triplet state (3T1). Triplet states have relatively long intrinsic lifetimes because the spontaneous conversion to the singlet ground state requires another Aspin flip@ which is a forbidden (low probability) transition. ISC is promoted by heavy atoms in the molecular structure or in the medium. The radiative decay of a triplet state leads to a weak light emission termed phosphorescence(PHOS) or Aafterglow@. Thermal agitation may excite a triplet state up to the higher energy of fluorescent state, followed by light emission with the fluorescence spectrum and the longer lifetime of the phosphorescence. This process is delayed fluorescence. Dye triplet states are chemically reactive owing to their relatively long decay lifetimes and the presence of unpaired valence electrons. Type I photosensitisation may be initiated by excited singlet states are uncommon. Fast electron transfer reactions between an unstable complex of an excited singlet state with the medium (an exciplex) and other proximate substrates have been identified. Type I photosensitisation initiated by a metastable triplet state is favored by the long decay lifetime and separation of the unpaired electrons ion different MO. In a Type II pathway, 1O2 is generated by Adownhill@ energy transfer from the sensitiser triplet state to molecular oxygen:
3
T1 (섹) + 3O2(씩) Y 1S0(센) + 1O2(센)The triplet energy of the sensitiser relative to the 1S0 ground state must exceed the 0.98 eV excitation energy of O2(1Dg+). Triplet states of many organic molecules are capable of generating 1O2. Quenching of a dye triplet state by molecular oxygen is a competing process:
3
T1 (세) + 3O2(씩) Y 1S0(셀) + 3O2(섹)where 3O2 denotes O2(3Sg G). Electron transfer may compete with 1O2 production by energy transfer:
3
T1 + 3O2 Y S+ + O2CGwhere S+ is the oxidized photosensitiser free radical and O2CG is the anion radical superoxide. Superoxide is the anion of the hydroperoxy (or perhydroxyl) radical HO2C with pKa = 4.4. It is an important form of Aactive oxygen@ with reactions that may differ from those of 1O2. Many cells, including red blood cells, contain the enzyme superoxide dismutase that catalyzes the of conversion of superoxide to hydrogen peroxide. Some photosensitisers generate both 1O2 by energy transfer from the triplet state and superoxide by electron transfer. Dye triplet states may react with many substrates other than oxygen. This process may either return the dye to its ground state (physical quenching) or initiate a chemical reaction (chemical quenching):
3
T1 + R Y 1S0 + R3
T1 + R Y S+ + Rred3
T1 + RY S G + Roxwhere Rred and Rox are quencher free radicals and S G is a reduced sensitiser free radical. In quinoid dyes SG is usually a semiquinone. A reduced sensitiser radical is another possible source of superoxide:
S
G + O2 Y S + O2CGQ
G + O2 Y S+ + O2CGPhotosensitisation can also generate hydroxyl radicals (OHC) by a Fenton reaction in which a metal ion is oxidized by hydrogen peroxide:
Fe2+ + H2O2
Y Fe3+ + OHC + OHG
3.1.1. Singlet oxygen quantum yield
: The singlet oxygen quantum yield (FD) is a key property of a photosensitising agent. This quantity is defined as the number of molecules of 1O2 molecules generated for each photon absorbed by a photosensitiser. Quantum efficiency is an equivalent term. The production of 1O2 by photosensitisation involves four steps: (A) Absorption of light by the photosensitiser; (B) Formation of the photosensitiser triplet state; the quantum yield of this process is the ISC efficiency or triplet yield (FT); (C) Trapping of the triplet state by molecular oxygen within its lifetime; the fraction of trapped triplet states in a given system is designated by fT ; (D) Energy transfer from the triplet state to molecular oxygen; the probability of this energy transfer is SD; the experimental value of SD is usually unity for those agents in which the fluorescence is not quenched by oxygen. Overall, FD = FT fT SD. Virtually all measurements of FD are scaled to a reference substance. Frequently employed standard values of FD in aqueous media are 0.79 for rose bengal, 0.52 for methylene blue, and 1.00 for fullerene C60. The published values of FD show considerable variations with the solvent, reaction conditions, and the measurement technique.3.2. Gaseous Discharge
: A gaseous discharge is established by passing an electric current through a gas or vapor. In the usual arrangement the electrodes are located inside a vessel containing the gas. There are many different types of gaseous discharge depending on the composition of the filling gas, the electrode configuration, and the operating conditions. Discharges are described as brush, corona, spark, glow, and arc. Each type of discharge has fairly well-defined characteristics, but the dividing line between one type and another is not always easy to determine. In the Geissler tube, dating to 1855, the application of high voltage to a low-pressure gas in a glass vessel generates a line spectrum characteristic of the filling gas. Practical examples are the neon tube and sodium vapor lamp. The intimate mixture of electrons and ions in the glowing region of the discharge constitutes a plasma. (A plasma is a unique state of matter in which electrons and ions interact collectively though long-range electromagnetic forces. Nearly all the visible matter in the universe exists in the plasma state, including the stars, auroras, and lightning.) A corona discharge is established at a higher gas pressure and higher voltage between a positive high-voltage point and a planar cathode This term originates from the appearance of the outermost region of the Sun's atmosphere, which consists of an extremely hot, low-density plasma. A corona converts to an arc at sufficiently high current densities. An arc is essentially a high-density plasma with Anegative@ electrical resistance, i.e., the current flow decreases with increasing applied voltage. A discharge can be established by injecting high-voltage electrons directly into a gas through a thin foil. In an electrodeless discharge the gas is excited by an external source of radiowaves or microwaves. Almost any type of discharge established in gaseous oxygen generates 1O2 as well as other species including oxygen atoms and ozone. Gas-phase studies of 1O2 reactions are based on the analysis of molecular emission spectra which typically have a complex electronic-vibration-rotation structure.The principal electronic transitions leading to 1O2 luminescence in the absence of quenchers correspond to: (A) radiative decay of 1O2:
O2(1
Sg+ ) Y O2(3Sg G) + hn (762 nm)O2(1
Dg+) Y O2(3Sg G) + hn (1269 nm);(B) Production of 1Sg+ by the interaction of two 1Dg+ states or energy pooling:
2 O2(1
Dg+) Y O2(3Sg G) + O2(1Sg+ ) Y O2(3Sg G) + O2(3Sg G) + hn (762 nm);2 O2(1
Dg+) Y 2 O2(3Sg G) + hn (634 nm)where hn indicates light emission. Physical and chemical quenching of 1O2 in the gas phase has been investigated for many agents. Some small molecules with high quenching rate constants include H2, N2, NO, N2O, CO, HCl, and NH3, C2H2, and C2H4. Chemical quenching can lead to complex reaction systems.
3.3.
Chemical reactions: Exothermic chemical reactions may generate 1O2 as a product. These reactions are often chemiluminescent owing to radiative decay of the 1O2 . The hypochlorite-peroxide reaction is well established:H2O2 + OCl
G Y 1O2 + ClG + H2OEnergy-rich compounds that generate 1O2 by thermal decomposition include phosphite ozonides:
(RO)3PO3 Y (RO)3PO + 1O2
and endoperoxides such as 9,10-diphenylanthracene peroxide (DAP):
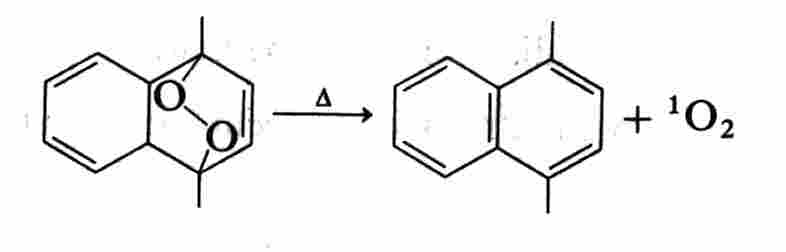
3.4. Enzymic reaction
s: Singlet oxygen luminescence has been reported for enzyme-catalyzed reaction systems, e.g., the myeloperoxidase-H2O2-ClGsystem, the lactoperoxidase-H2O2-BrGsystem, and the chloroperoxidase-H2O2-BrGsystem. The following mechanism dating to the 1970s was proposed for myeloperoxiodase:H2O2 + Cl
G Y OClG + H2OH2O2 + OCl
G Y 1O2 + ClG + H2O4. Detection and Measurement of Singlet Oxygen
The techniques of 1O2 detection and measurement depend on the generating system. Optical spectroscopy is widely employed in the laboratory and also for 1O2 in planetary atmospheres. Other techniques have been developed for condensed phases and the search for more sensitive and accurate procedures continues.
4.1. Singlet oxygen luminescenc
e: Luminescence is the Agold standard@ of singlet oxygen identification. Measurements in condensed phases are based on the 1269 nm luminescence emitted in the radiative decay of O2(1Dg+) and the 634 nm dimol emission. The weak emission intensities in chemiluminescent systems requires highly sensitive optical detection methods. Laser light sources have been employed for photosensitised systems. In the flash photolysis technique the 1O2 generating system is irradiated with a strong light flash which induces a luminescence pulse. The pulse height is proportional to FD and the decay of the luminescence leads to the 1O2 lifetime (tD). The value of tD is highly dependent on the medium, ranging from about 3 ms water to about 1000 ms in CF3Cl. There is an approximate correlation between the solvent-induced decay lifetime of 1O2 and the infrared absorption of the medium near 8000 cm-1 (1250 nm), suggesting that 1O2 decays by transferring its electronic excitation energy to vibrational energy of the solvent. An essentially unambiguous test for 1O2 in aqueous systems is based on the increase of tD .3 ms in H2O to .30 ms in D2O. Thus, the rate of reactions mediated by 1O2 are accelerated in perdeuterated solvents. The relative enhancement in aqueous systems is usually less than 10-fold owing to competing quenching reactions for 1O2 .4.2. Protective effects of additives
: Indirect tests for singlet oxygen are based on the inhibiting effects of an additive on the rate of a photochemical reaction. Azide ion (N3G) is a useful water-soluble agent. The protective effect of azide is attributed to physical quenching:1
O2 + N3G Y 3O2 + N3GOther frequently employed water-soluble quenching agents include 1,4-diazabicyclo-[2.2.2]octane (DABCO), ascorbic acid, and tryptophan. Some 1O2 quenchers used in organic solvents and phosopholipid membranes include cholesterol, a-tocopherol and b-carotene. The reaction of 1O2 with cholesterol leads to a stable product 3b-hydroxy-5a-hydroperoxy-D6-cholestene, while reactions of cholesterol with free radicals produce the corresponding A7a- hydroperoxide@ which can separated from the A5a-hydroperoxide@ by chromatography. The involvement of superoxide in a reaction system can be detected by adding superoxide dismutase that catalyzes the reaction:
2O2
CG + 2H+ Y H2O2 + O2A possible effect of hydrogen peroxide can be eliminated by adding catalase:
2H2O2
Y H2O + O2.Thus, inhibition of a reaction by low concentrations of superoxide dismutase plus catalase are indirect evidence for the involvement of superoxide. This test must be used with care because the enzymes react with 1O2.
4.3. Electron paramagnetic resonance
: A recent approach to singlet oxygen detection is based on electron paramagnetic resonance (EPR). EPR is a non-optical technique in which energy transfer between the intrinsic magnetism of unpaired electrons and an external magnetic field is measured with a sensitive microwave detection system. The magnetic interaction between an electron and the magnetic field corresponds to the energy difference between the Aspin-up@ and Aspin-down@ states:D
E = 2meBwhere me is the magnetic moment of the electron (the Bohr magneton) and B is the strength of the effective magnetic field. In EPR, a small sample is located in a microwave cavity that lies between the poles of a strong electromagnet. A transverse microwave field generated by a klystron is applied to the cavity which induces the spin transitions. The maximum power absorption takes place at exact resonance:
hf = 2meB
where f is the microwave frequency. In practical units, the resonance magnetic field of a free electron is 1.25 tesla (12,500 gauss) at the Q-band microwave frequency of 35 Ghz. An EPR spectrum is recorded by measuring the strength of the microwave signal when the magnetic field is swept over a small range. The EPR spectrum of a free electron consists of a single absorption "line" corresponding to transitions between the spin-up and spin-down states. (Each Aline@ consists of an adjacent Avalley@ and Ahill@ because the usual measurement technique leads to the first derivative of the absorption band.) The perturbing effects of nearby magnetic nuclei induces a substructure to EPR spectra (hyperfine interaction) because the magnetic fields of the nuclei add or subtract from the external magnetic field depending on the orientation of the nuclei in the external magnetic field. An adjacent atom of nuclear spin I leads to 2I + 1 lines. The EPR spectrum of an unpaired electron on an H atom consists of two equal intensity lines corresponding to I = 1/2. N equivalent adjacent protons lead to (N +1) EPR lines. 1O2 is a non-magnetic molecule and cannot be detected directly by EPR. However, the reaction of 1O2 with a stable molecule can generate a moderately long-lived free radical or a spin label whose structure as determined by EPR provides an unambiguous identification. The spin label 2,2,6,6-tetramethyl-4-piperidone (TEMP) has been employed as a spin label probe. The reaction of 1O2 with TEMP with leads to the free radical 2,2,6,6-tetramethyl-4-piperidone-N-oxyl (TEMPO):
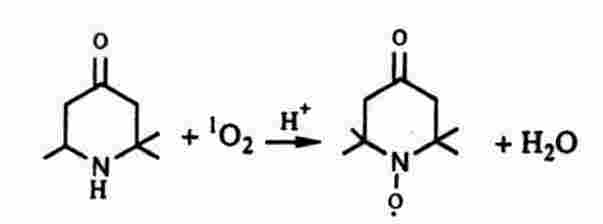
The EPR spectrum of TEMPO in ethanol consists of three equal intensity lines characteristic of a nitroxide radical owing to I = 1 for the 14N7 atom. Spin labels have been employed for detection of other active oxygen intermediates. Superoxide forms an unstable complex free radical with 5,5-dimethyl-1-pyrroline-1-oxide (DMPO) which has a characteristic four-line EPR spectrum. DMPO reacts with hydroxyl radicals to give a different four-line spectrum. EPR spin label measurements have been performed with strong steady light sources and under flash photolytic conditions.
5. Some Important Processes Mediated by Singlet Oxygen
5.1. Atmospheric airglow
: Airglow is a faint luminescence originating from 40 to 180 miles above the surface of the earth is emitted from the entire sky at all latitudes and at all times of the day and night. Atmospheric airglow has been measured from the ground, aircraft, balloons, and rockets. Airglow from the atmospheres of Venus and Mars have been measured using a high-resolution spectrometer on a telescope. The 1270 nm emission from O2(1Dg+) and the 762 nm emission of O2(1Sg+) are major components in airglow These species can be formed by direct absorption of UV radiation, e.g.:O2(3
Sg G) + hn Y O2( 1Sg+)O3 + h
n Y O2( 1Sg+) + O*where O* is an excited oxygen atom. Recombination of oxygen atoms is another mechanism:
O + O + M
Y O2( 1Sg+) + Mwhere M is a Athird body@. The complete analysis of airglow spectra is very complicated and many other reactions have been implicated.
5.2. Singlet oxygen reactions
: 1O2 reacts with many organic compounds including olefins, dienes, sulphides, aromatics, hetero-aromatics, terpenes, steroids, fatty acids, flavones, tetracyclines, vitamines, amino acids, proteins, nucleic acids, blood and bile pigments, and synthetic polymers Most of the reactions fall into three general classes. The Aene@reaction is a general type of reaction which is essentially a hydrogen abstraction and oxygen addition.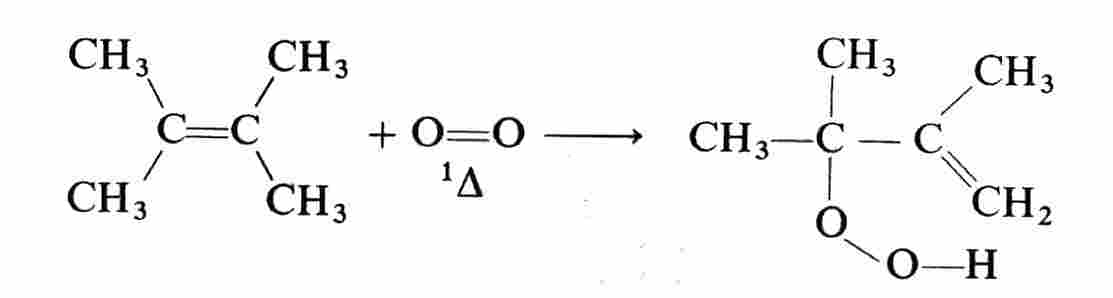
Cycloaddition is another general type of reaction. A 1,2-dioxetane is a typical product:
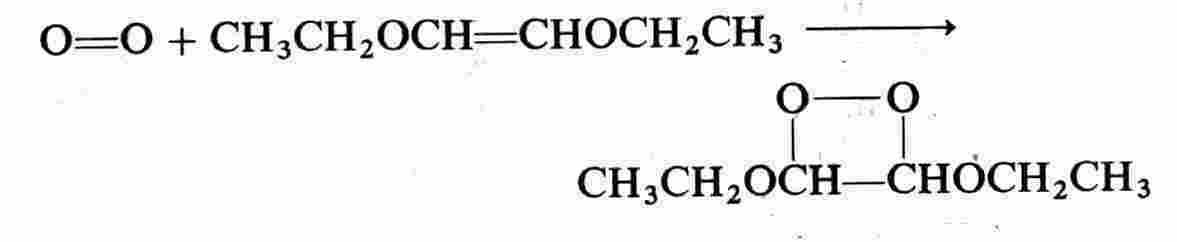
An endoperoxide is another type of cycloaddition product:
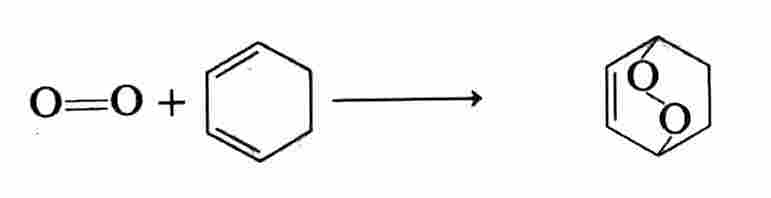
The products of these reactions are energy-rich and their thermal decomposition reactions are chemiluminescent. The endoperoxides of naphthalenes and anthracenes generally restore the parent compound plus 1O2. Oxygenation is another common reaction as exemplified by:
R - S - R + 1O2 Y R2SO
5.3. Lipid peroxidation
: Biological membranes are organized structures composed of a lipid bilayer incorporating structural and functional proteins, and frequently other agents including cholesterol, and a-tocopherol. Biomembranes regulate the flow of materials and information between different regions of cells and between a cell and its environment. Membrane damage induced by the attack of 1O2 on the lipid or protein moieties can be highly deleterious. Lipid structures are described in standard biochemistry texts. Briefly, a fatty acid is the simplest lipid consisting of a carboxylate group attached to a long hydrocarbon tail, e.g., palmatic acid is a saturated fatty acid: CH3(CH2)14COOH; oleic acid is an unsaturated fatty acid CH3(CH2)7CH=CH(CH2)7COOH. In a natural wax a fatty acid is joined to a long chain alcohol by an ester bridge, e.g., CH3(CH2)nCOO(CH2)mCH3. Phospholipids are the most common lipid constituents of biomembranes. The trifunctional alcohol glycerin forms the phospholipid skeleton: CH2OHCHOHCH2OH. Two of the three OH groups are joined to fatty acids by ester bridges. The third OH group is joined to a polar Ahead@ group by a triphosphate linkage. Common head groups are phosphatidylethanolamine, phosphatidylcholine, and phosphatidylserine. Another major class of membrane constituents is built on the long chain amino alcohol sphingosine: CH2OHCHNH2CHOHCH=CH(CH2)12CH3 . Many biomembranes contain cholesterol which promotes membrane fluidity. The existence of polar and nonpolar regions in a phospholipid molecules favor their assembly into various types of arrays in an aqueous environment. In a phospholipid monolayer the hydrocarbon head groups are aligned in the aqueous region and the fatty acid tails extend into the air. Some phospholipids form globular micelles in water with the head groups on the outside and the hydrocarbon tails in the interior. However, a bilayer is the favored structure consisting of two monolayers. The polar head groups are aligned on each exterior face and the hydrocarbon tails project into the interior in a head-tail-tail-head arrangement (Figure 3). In another type of
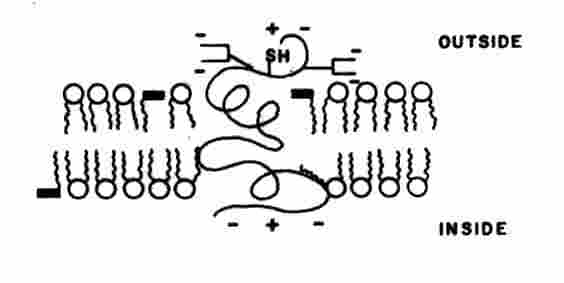
Figure 3. Representation of a biomembrane. The circles with twin tails depict phospholipids. The single tail entities depict
a-tocopherol. An intercalating membrane protein is shown. [Adapted from D. B. Menzel, In Free Radicals in Biology, Vol. II (Ed. W. A Pryor), Academic Press, New York, 1976]arrangement, the bilayer forms a spherical entity with an aqueous interior. This structure is referred to as a vesicle or liposome. Lipid peroxidation is initiated by molecular oxygen and active oxygen intermediates. The chemical alterations may be localized at a single site or in the form of lipid-lipid, lipid-protein, and protein-protein cross-links.
Lipid peroxidation is Aauto-catalyzed@ by intermediate radicals that propagate the initial damage via a chain reaction. This process requires electron transfer which is not likely for the Type 2 singlet oxygen pathway However, other active oxygen intermediates in a photosensitized system can generate a chain reaction. Lipid peroxidation mediated by 1O2 can be represented as:
LH + 1O2
Y LOOHwhere LH is an unsaturated lipid and LOOH is a lipid hydroperoxide. The actual mechanism is the Aene@ reaction. An oxidizing intermediate can initiate a chain reaction. The Type I photosensitization pathway mediated by a dye triplet state is:
3
T1 + LH Y LC + S G + H+L
C + 3O2 Y LOO CLOO
C + LH Y LC + LOOHwhere LC and LOO C are lipid free radicals. This system constitutes a chain reaction because LC generated in the first step is regenerated in the third step. The chain length (number of steps) is reduced if oxygen is not available and by the presence of antioxidants that reduce LOO C to LOOH . Some common anti-oxidants are a-tocopherol, b-carotene, and butylated hydroxytoluene (BHT). Superoxide dismutase and catalase are enzymic anti-oxidants.
5.4. Atmospheric pollution
: Most attention in atmospheric pollution and smo has been given to hydrocarbons, nitrogen oxides, and ozone. However, 1O2 generated in the lower atmosphere by the action of sunlight on polycyclic aromatic hydrocarbons (PAH) may be involved. PAH are a large class of organic pollutants released in the atmosphere by natural sources (e.g, volcanos, forest fires) and many types of man-made sources (e.g., automobile exhaust, industrial power generators, incinerators). More than a hundred PAH have been identified, of which anthracene, chrysene, benzo[a]pyrene, fluorenthane and 13 others are designated as priority pollutants by the U. S. Environmental Protection Agency. PAH absorb sunlight in the UV-A region (320-400 nm). Production of 1O2 by photosensitisation is favorable owing to the high triplet yields and long lifetimes of the triplet states. In addition to the direct reactions of 1O2 with biological substrates, PAH react with 1O2 to form unstable dioxetanes and endoperoxides which are potential candidates for biological damage.5. 5. Photohemolysi
s: Photohemolysis refers to the opening of red blood cell (RBC) membranes and release of hemoglobin induced by exposure to light. The earliest laboratory studies on photosensitsation of hemolysis were performed in conjunction with the prevalent disease of grazing animals termed hypericism. It has been known for long time that exposure of grazing animals to sunlight after ingestion of plants containing the natural pigment hypericin (HY) leads to inflammation, ulceration, and infection, and in severe cases, convulsions and death. The classic study of Blum in 1941 showed that photosensitised hemolysis by HY requires oxygen. This process exemplifies a photodynamic action. Photohemolysis of RBC in vitro is photosensitized by many other dyes and clinical drugs. This test is used as a predictor of agents that may photosensitise human skin. The involvement of 1O2 in the photohemolysis mechanism has been shown for halogenated fluorescein dyes, porphyrin derivatives, and some photodynamic therapy (PDT) drugs. Protoporpyrin IX (PpIX) is of particular interest because RBC of patients with the genetic disease erythropoietic protoporphyria (EPP) are readily photohemolysed in vitro. The colloid-osmotic model is the generally accepted mechanism of photohemolysis. According to this theory, photochemical damage to the RBC membrane promotes cation efflux, leading to swelling of the cells and eventually rupture. Studies with PpIX indicate that the lipid part of the RBC membrane and the anion channel membrane protein are photochemical targets, although the detailed relationship of the damage to hemolysis remains unresolved. Photohemolysis curves (fractional hemolysis vs light dose) generally have a sigmoidal shape with an incubation period. This type of rate kinetics is characteristic of a cooperative interaction. 1O2 is not involved in all cases of photosensitised hemolysis. Some photosensitisers are active under oxygenated and anoxic conditions (e.g., a-alkylamino-2-arylquinolinemethanol anti-malarial compounds, carprofen, griseofulvin ) and in other cases membrane-bound photolysis products are responsible for hemolysis (e.g., polytriptyline, chlorpromazine).5.6. Photodynamic therapy
: PDT is a combined light-plus-drug treatment for malignant tumors. The PDT drug Photofrin7 is approved in the U.S. for treatment of obstructing cancer of the esophagus and early stage cancer of the bronchus. Clinical trials are reported for tumors of the skin, brain, head and neck, urinary bladder, gastro-intestinal tract, and female genital tract. Some of these PDT procedures have received regulatory approvals outside the U.S. The most widely used PDT drug Photofrin7 is a water-soluble, red powder consisting of a mixture of metal-free porphyrins. The active constituent referred to as dihematoporphyrin ether (DHE) consists of covalent dimers or small oligomers of porphyrin units joined by ether and ester linkages. The ether structure is shown: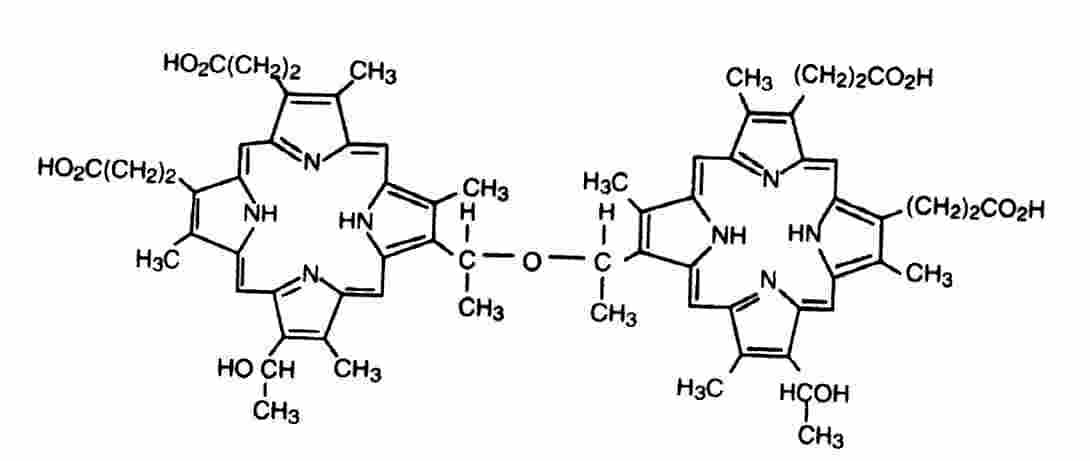
Variability in the composition of Photofrin7 does not appear to have a deleterious effect on its efficacy. Hematoporphyrin derivative (HPD) is the precursor of DHE which was first synthesized in the 1950s. HPD was originally used for tumor identification based on its yellow-red fluorescence after uptake in tumor tissues. The earliest clinical reports of its anti-tumor activity occurred in the 1970s. DHE localizes and is retained for many days in tumors anywhere in the body after intravenous administration. There is no therapeutic effect until DHE in tumor tissue is exposed to strong visible light, usually in the red region of the spectrum. The light exposure induces necrosis followed by sloughing of the necrotic tissue and regrowth of normal tissues. The putative action mechanism in PDT is that 1O2 generated by energy transfer from the DHE triplet state to tumor oxygen initiates lipid peroxidation in the endothelial cells of the small blood vessels supplying the tumor cells. This process shuts down the tumor oxygen supply and induces the observed necrosis. Direct cancer cell killing may be involved as well. Interestingly, oxygen is required for the initial photochemical reaction and its depletion initiates the clinical response. The skin of patients who have been injected with HPD and DHE may be highly sensitive to strong sunlight for several weeks or longer. Many potential new PDT drugs have been investigated including porphyrins, chlorins, and phthalocyanines. The drug properties deemed favorable for PDT include synthetic purity, effectiveness at far-red and near infrared wavelengths where tissues are more transparent, and short-term photosensitization of the patient=s skin. Several of the new drugs have progressed to clinical trials. These include benzoporphyrin derivative monoacid ring-A (BPD-MA), lutetium texaphyrin (LUTRIN7), and 5-aminolevulinic acid (ALA). ALA differs from the other PDT drugs because this colorless compound is the precursor of the active PpIX which is formed by in vivo metabolic processes. The action mechanism for these agents may not be the same as DHE owing to different pharmakokinetics.