Hypericin-mediated impact of UV and VL on collagenesses in collagen
Dido Yova1, Vladimir Hovhannisyan2 , Theodossis Theodossiou1 and Vladimir Gukassyan2
1National Technical University of Athens, Department of Electrical Engineering and Computing Biomedical Optics and Applied Biophysics Laboratory, Zografou Campus, 157 80 Athens, Greece
2Laserayin Tekhnika Research Institute, 21 Shopron st., Yerevan 375090, Armenia
Keywords: Collagen, gelatin, hypericin, chlorin e6, Nd:YAG laser, fluorescence quenching, photobleaching ABSTRACT
The emission and excitation spectra of collagen were investigated in the UV and visible regions. The existence of several types of chromophores throughout these spectral regions was observed. It was shown that laser irradiation at both 355 and 532 nm resulted in photobleaching of collagen fluorescence. The photoinduced effect of hypericin (HYP), a polycyclic quinone, on collagen was also studied. Addition of HYP aqueous solution to collagen produced quenching, red-shift of the maximum and broadening of the spectral form of its fluorescence. These effects increased with increasing concentrations of HYP. After HYP addition and laser irradiation at 355 nm, collagen fluorescence, decreased in a spectrally inhomogeneous manner. These processes of photodestruction of collagen fluorophores proved to be one photon effects. The effect of Chlorin e6 on collagen was also investigated and proved to be dissimilar to that of HYP and not indicative of profound spectral alterations. INTRODUCTION
Many recent works showed that the ozone layer reduce brings to the
increase of UV impact. From the other side, it has been reported also about the VL
impact on collagen, which is the most widespread of structural proteins in higher
vertebrates. It comprises about 6% of body weight in mammals and the main means of their
structural support. Collagen has a molecular weight of about 300.000 and its molecules
consist of three peptide chains that form a rod shaped, triple helix. The studies of
collagen in cell and molecular biology reveal that it is an essential component of
extracellular matrix in organism but also performs important physiological functions in
cell migration, proliferation, differentiation and growth. Many kinds of diseases are
related to pathological changes of collagen. The fluorescence of collagen in the UVA and
visible spectral regions has been investigated in many papers [1,2] and several attempts have been made to clarify the nature and
origin of it [3,4]. Destruction
of type I collagen under UVA (335-400 nm) and broadband solar simulating radiation
(290-400 nm) has also been demonstrated [5]. In this
reference the existence of at least four photolabile fluorescent chromophores was shown,
some of which absorb electromagnetic radiation in the region between 300 and 400 nm. In
this manner the alterations to collagen, the main connective protein in human skin under
chronic exposure to solar UV is well documented [5-7]. These
studies have demonstrated collagen cross - linking, destruction of tyrosyl and
phenylalanyl residues, conformational change with concominant chain degradation and
suppression of fibril formation. Collagen may also be related with tumorigenesis and
metastasis of neoplastic cells. In this context the investigation of the interaction of
collagen with photosensitisers and light is of high importance.
Since at the present time some ointments are used, containing aromatic compounds which
absorb in the VL band as well, it's interesting to study photosensitized collagen
photochemical and photobiological reactions, initiated by VL. At the VL absorbing by
aromatic compounds, energy flow is possible in the photosensitizer molecule - protein
direction, activating the latter and forming chemically active radicals, which could
interact with skin proteins as well as induce various biological effects. It's well known
that hypocrilin and hypericin photosensitizers permeate collagen fibrills easily and can
effectively interact with collagen's various aminoacids residues.
In the current work we investigated laser-induced photoprocesses in collagen and prove
that even visible light (532 nm) can infer irreversible damage to collagen. Different
kinds of photosensitisers, both endogenous and exogenous can enhance induced photochemical
processes in collagen.
Hypericin (HYP), a polycyclic aromatic quinone is a natural pigment synthesised by plants
of the Hypericum family and some insects fungi and protozoa and has recently received
increasing attention, due to its high toxicity against viruses, including HIV, under the
presence of light [8,9] and its
anti-tumour photo-activity [10] due to a combination of
mechanisms. These include singlet oxygen production (FD»0.5),
superoxide anions formation [11] and excited state charge
transfer processes [12, 13].
The photophysical properties of HYPseem to be strongly dependent on the surrounding
medium. In this context its fluorescence quantum yield is (FF»0.2) in polar organic solvents, but drops quite steeply (FF»0.02) in water and most
likely organic apolar solvent environment, due to extensive aggregation [14].
These properties have caused HYP to be considered as a potent dye photosensitiser for the
photodynamic therapy of cancer and other diseases [15] in
the current study the interaction of collagen with UV and visible laser radiation as well
as with HYP was investigated, to elucidate its photophysics and photochemistry. MATERIALS
AND METHODS
Collagen from bovine Achilles tendon and gelatin (Fluka Biochemica 27662
and 48724 respectively) were investigated. HYP derived from Hypericum Perforatum
according to the standard gel column chromatography procedure [9],
was used as sensitizer in the form of water solution. Chlorin e6 (CHL) obtained
from Institute of Molecular and Atomic Physics, Minsk, Belorus was also used as a
sensitiser in the form of water solution.
Collagen was studied in dry form as well as in aqueous environment. Gelatin was studied in
dry form.
Sample irradiation and excitation was carried out with the use of a computerised laser
system based on a Q-switched pulsed Nd:YAG laser emitting at 1064 nm (Fig. 1). 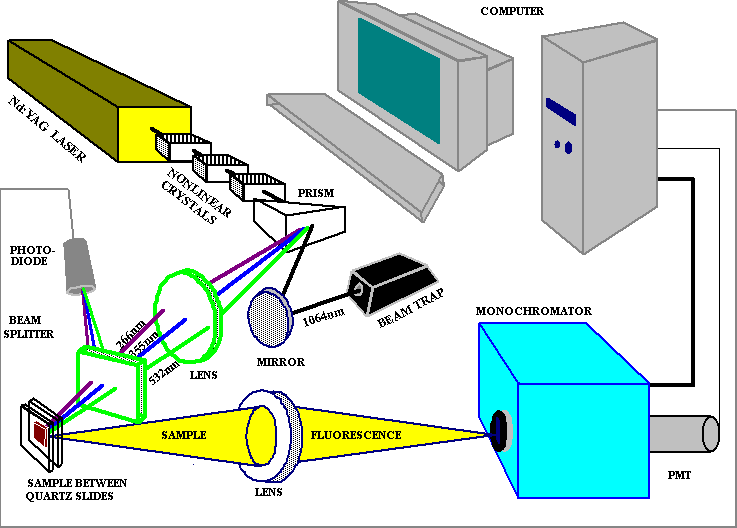
Fig. 1. Experimental setup for investigation of laser inducedphotoprocesses.
The fundamental frequency was converted into the second and third
harmonics using LiJO3 nonlinear crystals. The output laser radiation
parameterswere selected as follows: for the second harmonic (l
=532 nm) -pulse energy E=15 mJ; for the third harmonic (l =355
nm) -pulse energy E=5 mJ. The pulse duration was 12 ns and the repetition rates used, were
5 and 10 Hz. The intensity of laser radiation at 532 and 355 nm was decreased with the use
of neutral density glass filters with 50% and 75% absorption at these wavelengths. The
samples were placed between two thin quartz slides with virtually no detectable
fluorescence at 266, 355 or 532 nm excitation. The spot of the laser beam focused on the
approximately 3 by 3 mm and 0.2mm thick samples, was an ellipse of about 1.5mm by 2 mm.
The emission from the object was collected by a lens at 90°
and delivered to the slit of a monochromator (MDR-23 LOMO, St. Petersburg), which together
with a photomultiplier tube (FEU-79) was used for detection and spectral analysis of the
fluorescence signal. A part of the laser radiation was directed to a photodiode for
normalisation of the excitation radiation. A personal computer and a 2-channel
analog-to-digital converter were used for system control and automatic data collection and
processing. The spectral resolution of the system was less than 3 nm.
Excitation and emission spectra were recorded on a Perkin-Elmer Hitachi MPF 43B
spectrofluoro-meter. RESULTS
Using the experimental setup described above, we obtained the fluorescence spectra of dry collagen at three excitation wavelengths 266, 355 and 532 nm (Fig. 2). In spectrum 3 of Fig. 2 the fluorescence below 550 nm was cut with the use of glass cut-off filter.
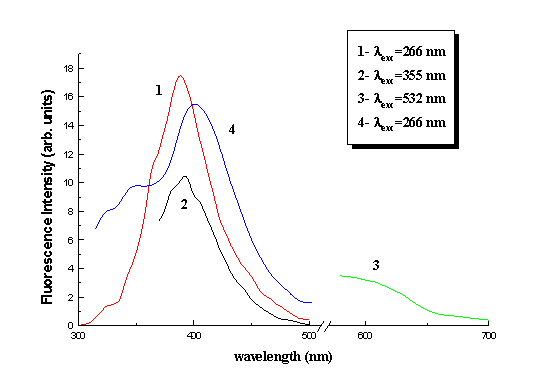 |
Fig. 2. Fluorescence spectra of dry collagen (1-3) and gelatine (4)
under laser excitation.
It must be noted that nanosecond laser excitation in combination with our fast
registration system allow for the registration of fluorescence but not phosphorescence.
This was verified with the measurement of luminescence lifetime which was found to be less
than 80 ns. In Fig. 2 one can see that the fluorescence spectra are strongly dependent on
excitation wavelength. From excitation at 266 nm it is directly obvious that the
characteristic fluorescence of tyrosine (303 nm) and pnenylalanine (290 nm) residues is
absent. This fluorescence spectrum has maximum at 387 nm for collagen. Excitation of
collagen at 355 nm produced a fluorescence spectrum less broad than the one at 266 nm and
with its maximum shifted to 395 nm. The presence of a shoulder can be seen at 405 nm.
Finally excitation at 532 nm produced a fluorescence spectrum without structure and
distinctive maximum. In Fig. 2 the fluorescence spectrum of gelatin under 266 nm
excitation also appears. The maximum of gelatine fluorescence is at 402 nm.
Excitation and fluorescence spectra of dry collagen were obtained by the use of a
Perkin-Elmer standard spectrofluorometer. The acquired fluorescent spectra were in full
agreement with the ones obtained by laser excitation. The excitation spectra were taken at
383, 405, 440 and 480 nm emission wavelengths (Fig. 3). The corresponding maxima are at
338, 343, 372 nm, while for emission at 480 nm there is no maximum but monotonic increase
almost up to emission wavelength. 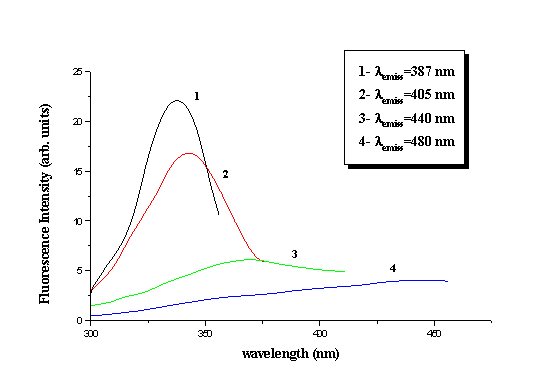
Fig. 3. Excitation spectra of dry collagen.
The addition of bidistilled water to collagen did not produce any effects on the spectral
profile of its fluorescence. Addition of HYP solution to collagen produced quenching of
its fluorescence. This quenching was not spectrally homogeneous, as it was stronger at the
short wavelength region and considerably weaker at the long wavelength region of the
spectrum. More specifically there was quenching, red-shift of the maximum and broadening
of the spectral form increasing with increased concentrations of HYP. The effects of
different concentration HYP solutions on collagen can be seen in Fig. 4. After addition of
0.03 ml of HYP 10-5M solution, the fluorescence maximum of collagen shifted
from 395 to 420 nm under 355 nm laser excitation. More shifting of this maximum to 430 nm
was observed after the addition of HYP solution of the same amount and concentration.
After each HYP addition the optical density of the sample at 355-500 nm was increased by
an estimated value of 10-2 and consequently absorption of the excitation
radiation or collagen fluorescence by HYP can be considered negligible. During water
evaporation from the collagen sample, more effective quenching and shifting of its
fluorescence was observed (Fig. 4). This effect can be explained by the substitution of
water molecules resident in collagen fibrils with HYP molecules, resulting into more
intensive interaction of HYP with collagen.
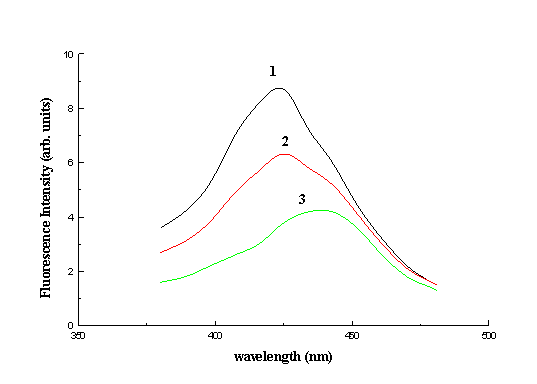
Fig. 4. Fluorescence of collagen sensitised by 1) 10-5M
hypericin solution, 2) 2x10-5M hypericin solution and 3) 10-5M
hypericin solution after partial evaporation of water.
The same experiments were performed with CHL solution of the same concentration and amount
as the HYP. It was visually obvious that CHL could not penetrate into collagen fibrils and
thus was only able to interact with the sample’s surface in contrast to HYP.
Consequently there was no spectral shifting or quenching of collagen fluorescence and
there was no registration of CHL fluorescence from the collagen sample. However CHL
fluorescence was very well observed from the solution surrounding the collagen sample.
Besides photophysical effects in collagen and gelatin, laser irradiation of samples
revealed the existence of photochemical processes of potentially high importance from a
biological point of view. Laser irradiation at 355 nm on dry collagen and gelatin as well
as collagen in aqueous environment samples induced pronounced photobleaching effects on
the macromolecules. This effect took place in a more or less spectrally uniform manner.
After interruption of irradiation neither recovery or further decrease of the fluorescence
intensity was observed. The absence of any dark effect is obvious while decrease of
fluorescence continued after re-irradiation. The same experiment was performed with laser
irradiation at 532 nm, to investigate the existence of photochemical processes induced to
collagen by visible light because of its observed absorption and fluorescence in this
spectral region. The result of laser irradiation of dry collagen at 532 nm with time can
be seen in Fig. 5. It is immediately obvious that there is photobleaching and that the
effect is dose and pulse intensity dependent. With an intensity increase by a factor of 2,
3 and 4 the initial rate of photobleaching increased by 1.65, 2 and 2.23 correspondingly
(Fig. 6). It follows that this effect is a one-photon effect and the population of the
first excited electronic level is saturated with continuous increase of irradiation
intensity. After irradiation of the HYP sensitized collagen sample in the UV or visible
region a profound decrease of intensity and alteration of the fluorescence spectral form
takes place as shown in earlier work [16]. This alteration increases with time of
irradiation. The maximum of collagen fluorescence after photosensitization and 10 min of
irradiation at 355 nm, shifted to 440 nm. In Fig. 6 one can note the increase of
photochemical destruction of collagen fluorophores after HYP sensitisation and irradiation
at 532 nm. 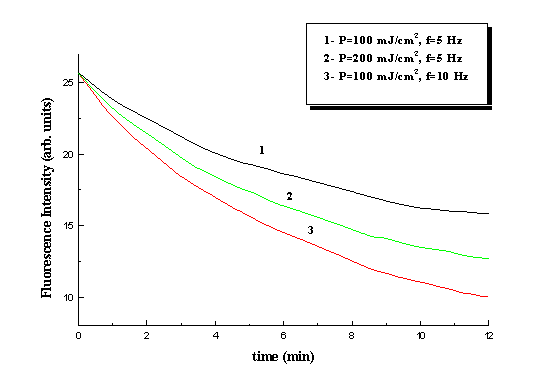
Fig. 5 Kinetics of collagen fluorescence photobleaching under 532 nm laser irradiation at different pulse energy density and repetition rates. lfl=585nm
. 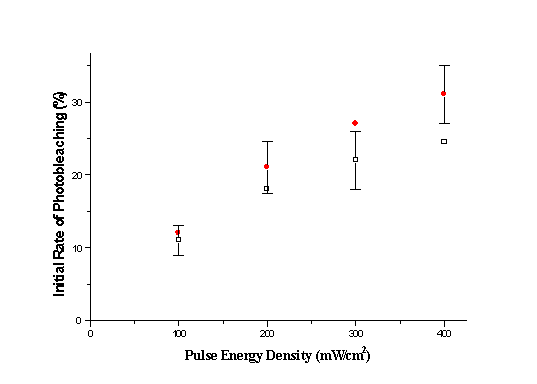
Fig. 6. Initial rate of sample photobleaching at 532 nm laser irradiation. Hollow squares denote the effect on pure collagen, while circles denote photobleaching of hypericin sensitised collagen. lfl=585nm. DISCUSSION
There are numerous suggestions in literature with regard to the nature of long wavelength collagen fluorescence. Long wavelength photoluminescence of collagen and several other proteins in with no tryptophan content was at first explained as phenylalanine phosphorescence because their spectral forms coincide. However it was consequently demonstrated [3] that collagen luminescence in the 400 - 700 nm region, has a lifetime of the order of 10 ns. Our work combining nanosecond laser excitation and fast registration corroborated this result. In this context phosphorescence can be excluded as a possible explanation of collagen fluorescence in the visible spectral region. In [3] the authors suggest that collagen fluorescence is of excimer nature and is mainly attributed to phenylalanine residues as the fluorescence of collagen coincides with that of phenylalanine in powder form. In our work, nevertheless, long wavelength collagen excitation spectra not characteristic of monomer phenylalanine were registered. Hence it is safe to deduce that excimer formation cannot be the main mechanism for the explanation of long wavelength (500-700 nm) absorption and fluorescence of collagen. The dependence of emission spectra on excitation wavelengths and excitation spectra on emission wavelengths, suggests the existence of several chromophores that absorb and fluoresce both in UV and visible spectral regions. Some age related collagen chromophores were isolated and identified as pentosidine and pyridinoline [2,4]. However both these chromophores absorption and fluorescence maxima, lie below 400 nm. Our experiments indicate the existence of chromophores that absorb and fluoresce in the visible spectral range. Moreover our results show that these chromophores can very easily be destructed under visible laser light irradiation and and they perhaps participate in important photochemical processes in collagen. Since this photobleaching was shown to be a one-photon effect, it is possible to be the result of long time exposure even to low intensity light source. In our experiments 600 J/cm2 at 532 nm, were sufficient to induce a 50% photodegradation. The photoproducts of this chromophore destruction can react with the chain or aminoacid residues and induce important biological effects. The result of collagen fluorescence spectrally non-uniform quenching by HYP (with shifting towards longer wavelength), also verifies the exsistance of more than one chromophores in collagen. HYP can very easily come into close range and interact with collagen fluorophores responsible for its short wavelength fluorescence while its quenching effect on fluorophors emitting at longer wavelengths is not as profound. The sensitisation of collagen with hypericin increases the efficiency of photochemical processes in collagen under both UV and visible irradiation. Although photobleaching of pure collagen has a uniform character, in the case of HYP sensitised collagen there is also shifting of the fluorescence towards longer wavelengths. The mechanism responsible for fluorescence decrease and maximum shifting in this case is possibly very similar to he one of fluorescence quenching after addition of HYP to collagen. Addition of hypocrellin to collagen solution [17] produced results similar to ours, namely quenching of collagen fluorescence and shifting of its maximum. The authors show that the main mechanism responsible is charge transfer but not energy transfer. Since HYP has very similar structure and properties to hypocrellin, it is also our belief that the main mechanism responsible for HYP sensitised collagen fluorescence decrease is mainly due to charge transfer between HYP and collagen chromophores. CONCLUSIONS
The photobleaching of collagen fluorescence under UV (355 nm) and visible (532 nm) laser light irradiation was observed. A photoinduced change of the fluorescence spectral profile of HYP of collagen has been observed after irradiation at 355 and 532 nm. The effect is HYP concentration dependent and requires irradiation. We believe that photobleaching is directly associated with the destruction of photolabile chromophores. In the case of visible light irradiation, collagen chromophore species that absorb and fluoresce in that spectral egion are affected. The above mentioned results are of a preliminary nature. More detailed experiments are to be performed for detailed quantitative results and definition of the nature of collagen chromophores in the visible spectral region. We believe that the present studies of collagen fluorescence and photochemical behaviour are of utmost biological importance. The observation of photobleaching under visible light irradiation, may affect the contemporary philosophy of sun protection which is focused in screening the UV electromagnetic radiation while maximal sun irradiation is around 500 nm.
ACKNOWLEDGEMENTS This work was supported by NATO research grant
REFERENCES
- E. A. Burshtein, Autoluminescence of Proteins (Nature and Applications). Biofizika, Vol. 7 Moscow: VINITI AN SSSR, 1977.
- A. Uchiyama, T. Ohishi, M. Takahashi, K. Kushida, T. Inoue, M. Fujieand and K. Horiuchi, “ Fluorophores from aging human cartilage” , Journal of Biochemistry, Vol. 110, pp. 714-718, 1991.
- A. S. Volkov, S. E. Kumekov, E. O. Syrgaliev and S. V. Chernyshov, “ Photoluminescence and anti-Stokes emission of native collagen in visible range of the spectrum” , Biofizika, Vol. 36, pp. 770-773, 1991.
- D. Fujimoto, K. Akiba and N. Nakamura, “ Isolation and characterisation of a fluorescent material in bovine Achilles tendon collagen” , Biochemical and Biophysical Research communications, Vol. 76, pp. 1124-1129, No. 4, 1977.
- J. M. Menter, J. D. Williamson, K. Carlyle, C. L. Moore and I. Willis, “ Photochemistry of type I acid soluble calf skin collagen: Dependence on excitation wavelength” , Photochemistry and Photobiology, Vol. 62, pp. 402-408, No. 3, 1995.
- D. R. Cooper and R. J. Davidson, “ The effect of ultraviolet irradiation on soluble collagen” , Biochemistry Journal, Vol. 97, pp. 139-147, 1965.
- K. Sudoh and H. Noda, “ UV irradiation of collagen and its effect on fibril reconstruction” , Connective Tissue Ressearch, Vol. 1, pp. 267-274, 1972.
- S. Carpenter and G. A. Krauss "Photosensitisation is required for inactivation of equine infectious anaemia virus by hypericin", Photochem. Photobiol., 53, pp. 169-174, 1991.
- D. Meruelo, G. Lavie and D. Lavie, "Therapeutic agents with dramatic antiretroviral activity and little toxicity at effective doses: Aromatic polycyclic diones hypericin and pseudohypericin", Proc. Nat. Acad. Sci. USA, 85, pp.5230-5234, 1998.
- H. Koren, G. M. Schenk, R. H. Jindra, G. Alth, R. Ebermann, A. Kubin, G. Koderhold and M. Kreitner, " Hypericin in phototherapy", Photochem. Photobiol., 36, pp. 113-119, 1996.
- J. M. Fernandez, M. D. Bilgin and L. I. Grossweiner, "Singlet oxygen generation by photodynamic agents", J. Photochem. Photobiol., B37, pp. 131- 140, 1997.
- D. S. English, K. Das, K. D. Ashby, J. Park, J. W. Petrich and E. W. Castner, "Confirmation of Excited-State Proton Transfer and Ground-State Heterogeneity in Hypericin by Fluorescence Upconversion", J. Am. Chem. Soc., 119, pp. 11585-11590, 1997.
- T. A. Wells, A. Losi, R. Dai, P. Scott, S. M. Park, J. Golbeck and P. S. Song, "Electron Transfer Quenching and Photoinduced EPR of Hypericin and the Ciliate Photoreceptor Stentorin", J. Phys. Chem. ,A101, pp. 366-372, 1997.
- A. Losi, "Fluorescence and Time-Resolved Photoacoustics of Hypericin Inserted in Liposomes: Dependence on Pigment Concentration and Bilayer Phase", Photochem. Photobiol., 65, pp 791-801, 1997.
- B. W. Henderson and T. J. Dougherty, " How does photodynamic therapy work?", Photochem. Photobiol. 55, pp. 145-157, 1992.
- D. Yova, T. Theodossiou and V. Hovhannisyan, “ Photoinduced effect of hypericin on collagen and tissue with high collagen content” Laser - Tissue Interaction and Tissue Optics. Proc. SPIE 1998 in print.
- Y. H. Li, J. C. Yue, G. P. Cai, “ Fluorescence Characterization of Type I Collagen from normal and Silicotic Rats and Its Quenching Dynamics Induced by Hypocrellin B” , Inc. Biopoly., Vol 42, pp.219-226, 1997.