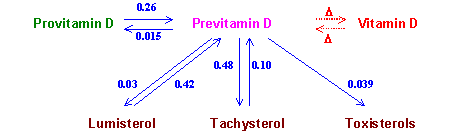
Figure 1. Drawing of the scheme of vitamin D synthesis. Numbers denote the photoconversions quantum
yields.
THE NEW METHOD OF UV DOSIMETRY
BASED ON AN IN VITRO MODEL OF PREVITAMIN D PHOTOSYNTHESIS
Irina P. Terenetskaya
Institute of Physics, National Academy of Sciences of Ukraine
252022 Kiev-22, Ukraine
e-mail: teren@iop.kiev.ua
Abstract.
A new method of biological dosimetry of UVB radiation based on an in vitro model of previtamin D photosynthesis is presented. The important role of vitamin D synthesis upon UV solar radiation is briefly reviewed. Distinctive features and extended capabilities of the method are discussed in detail with reference to the results of laboratory and field tests.
Keywords: UV radiation, biological dosimetry, vitamin D synthesis, spectrophotometric analysis, mathematical modeling.
1. Introduction
Although excessive exposure to sunlight is liable to cause skin cancer and premature aging of the skin, obviously these acute and chronic effects only occur upon excessive exposure to UV radiation. This suggests that the intensity of biologically active UVB (280-315 nm) solar radiation at the earth surface needs permanent control.
Two complementary methods of measuring UV radiation are spectroradiometry and biological dosimetry [1]. Spectroradiometers provide the most detailed spectrally resolved information on solar UV in physical units, but they are expensive bulky instruments requiring regular calibration. To give a biological dose, the spectrum measured by a spectroradiometer is weighted (integrated over the wavelengths) according to the action spectrum of a specific biological effect.
An alternative approach involves biodosimeters that use the simplest biologic systems (bacteria, spores, biomolecules) and measure the integrated biological dose directly weighting UV radiation by the absorption spectrum of a molecule whose excitation is a basis for a photobiologic effect. Biodosimeters are cheap small devices requiring no external power and thus are widely accepted for both natural and artificial UV dose control. However, for the most part biodosimeters have a number of disadvantages given below:
The method of UV biodosimetry based on an in vitro model of vitamin D synthesis [2] is free from
the disadvantages mentioned above:
These basic features of the method are discussed below.
2. Vitamin D synthesis is beneficial effect of solar UVB radiation
Health promoting properties of sunlight have been recognized from the beginning of civilization although beneficial influence of solar UV radiation has been discovered by Danish doctor Niels Ryberg Finsen only by the turn of the 19th century. Vitamin D synthesis is the most well known and well documented beneficial effect of solar UVB radiation. There is a good reason to believe that synthesis of vitamin D initiated in mammal skin by the UV photons was essential for the formation and evolution of the vertebrate skeleton and just was primarily responsible for the vertebrates beginning on the Earth.
History of vitamin D discovery is intimately linked to the history of industrialization in Northern Europe when rickets (commonly known as "English disease") has been widely occurred as a consequence of air polluted sunless environment in industrial centers. Later on it was found that UVB irradiation of rachitic children results in remission of the disease rickets.
Now it is acknowledged that vitamin D, traditionally perceived as an antirachitic vitamin essential for regulation of calcium homeostasis, is responsible for a wide array of biological processes via mechanisms analogous to classical steroid hormones [3]. In general, there is an opinion [4] that effects of UVB sunlight and equivalent artificial light on physiological and behavioral processes are probably mediated, in large part, through the skin - vitamin D - endocrine system.
All higher vertebrates have an endogenous mechanism involving solar UVB radiation that initiates vitamin D synthesis. Studies in plants have also demonstrated that at least some plants, when exposed to UVB radiation, possessed antirachitic activity [5]. Holick et al. [6] evaluated the vitamin D synthetic capacity of zooplankton, amphibians, reptiles, avian species and aquatic mammals and found that they all had the capacity to produce vitamin D upon exposure to sunlight.
Vitamin D synthesis consists of two basic stages. At the first stage previtamin D is formed from initial provitamin D photochemically, and at the second stage it converts into vitamin D by thermoisomerization. Despite the terminology vitamin D is employed in the general sense, there are two chemical species of vitamin D. Vitamin D2, or ergocalciferol (C28H44O), is synthesized upon UVB irradiation from ergosterol (provitamin D2) mostly in plants. Vitamin D3, or cholecalciferol (C27H44O) is photochemically produced in animals from 7-dehydrocholesterol (7-DHC, provitamin D3). It is significant, that basic monomolecular isomerizations of the two steroid species occur in perfect analogy.
A steep decrease in provitamin D absorption spectrum within the interval 280-310 nm causes high sensitivity of vitamin D synthesis to the spectral composition of UVB radiation. Despite sunlight has long been recognized as the principal source of vitamin D3 for humans, dramatic influence of seasonal and latitudinal changes of solar UVB radiation on vitamin D3 synthesis (in vivo and in vitro) has been revealed [7]. From this fact high sensitivity of the process to ozone layer depletion can be deduced.
Another important factor affecting vitamin D synthesis is air pollution. Growing incidence of rickets in children has been observed in Mexico City in period 1990-1992 as a result of severe air pollution [8]. Positive correlation of breast cancer mortality with low sunlight levels and high air pollution levels was found in major U.S. cities, in Italy and in the former Soviet Union [9].
These data testify that the problem of the in situ control of solar UV radiation in respect to its ability to initiate vitamin D synthesis deserves much concentrated attention, and adoption of an in vitro model of previtamin D photosynthesis has great potential to attain these ends.
2. Materials and method
2.1. Spectral analysis
The photochemical stage of previtamin D photosynthesis is considerably complicated by the previtamin D side reversible and irreversible photoisomerizations (Fig.1). Since the absorption spectra of the photoisomers overlap in the same spectral region (Fig.2), UV irradiation of starting provitamin D produces the multicomponent mixture and is accompanied by the transformation of provitamin D absorption spectrum. This opens the way of UV dosimetry by the spectral monitoring in a manner like
|
|
|
Figure 1. Drawing of the scheme of vitamin D synthesis. Numbers denote the photoconversions quantum yields. |
polysulphone or uracil dosimeters. Figure 3(a) illustrates the transformation of provitamin D absorption spectrum upon irradiation by solar simulator (xenon arc lamp with cellulose di-acetate cutoff filter). By this means "Vitamin D" biodosimeter can operate in the "spectral mode", and the decrease of absorbance at
lmax = 282 nm depending on the exposure time can be evaluated in terms of biological dose after calibration procedure.|
|
|
Figure 2. Absorption spectra of provitamin D and its main photoisomers (Pro – provitamin D, Pre – previtamin D, T – tachysterol, L – lumisterol) |
Considerable gain in the accuracy and enhancement of capabilities of the method can be achieved in a "concentration mode" when the photoisomer concentrations are determined for the every exposure time. In principle, the dose dependence of each photoisomer can serve as a measure of the UV effect, but provitamin D decay and previtamin D accumulation are the most suitable. The decay of provitamin D is exponential in wide range of doses and has analogy to biological response of many biodosimeters in the form of some damage caused by UV radiation. The kinetics of previtamin D accumulation is directly associated with the biological endpoint of interest, e.g. vitamin D synthesis. This kinetics is more complicated (Fig.3b), it is linear up to C» 10%, then concentration of previtamin D reaches the maximum, and further slowly decreases due to the side photoconversions.
|
|
|
|
Figure 3.a) Transformation of provitamin D absorption spectrum upon irradiation by the solar simulator; b) photoisomer concentration kinetics ("Sum" is the total concentration of Pro, Pre, T and L). |
|
The biological dose is determined by measuring provitamin D and previtamin D concentrations depending on the exposure time. Method of high performance liquid chromatography (HPLC) is commonly used to perform concentration analysis, but it is quite expensive, time consuming and thus is not convenient for UV dosimetry. Considerable progress has been achieved with the development of spectrophotometric analysis whereby the absorption spectra are recorded by a spectrophotometer at different exposure times and then the photoisomer concentrations are derived from the spectra by computer processing [10]. In such a manner the in situ control of biological UV dose can be provided. (For this in mind elaboration of portable "VitaD" biodosimeter with a built-in computerized spectrophotometer is planned).
"Vitamin D" biodosimeter uses low concentrated solution of provitamin D (ergosterol or 7-dehydrocholesterol) in ethanol (C = 0.002%) irradiated in quartz cuvettes (4 cm ´
1 cm ´ 0.5 cm) laid horizontally and provided with a Teflon stopper. (Global UV radiation can be monitored using spherical quartz cuvettes 2 cm in diameter). Before an exposure the absorption spectrum is recorded by a spectrophotometer within 230-330 nm. For the measurement of a daily accumulated dose the solution is exposed during the day and the absorption spectra are monitored every 1 hour, but to obtain day-profile data (with 0.5-1 hour time resolution) a number of fresh solutions of Pro should be exposed one by one during the day. If necessary, the irradiated solutions can be stored in a refrigerator up to several months without significant changes in the absorption spectra.Although the second,- thermal,- stage of vitamin D formation is useless for UV dosimetry, the temperature dependence of the Previtamin D<=>Vitamin D conversion sets the limits of the exposure time when the thermoreaction can be neglected. It was found [11] that at 20C, in vitro, it took 20 hours to form 10% of vitamin D from previtamin D, and Pre<=>D equilibrium was established in 30 days. At 40C these times reduce to 2,3 hours and 3,5 days correspondingly. Consequently, in most cases using "Vitamin D" biodosimeter we can exclude the thermoreaction from consideration.
2.2. Mathematical model and action spectrum.
To date the photoreaction scheme of previtamin D photosynthesis is known in sufficient detail mainly due to comprehensive studies of E. Havinga and co-workers [12,13]. Representing a biological process, the reaction of previtamin D photosynthesis at the same time can be treated as a physical system with known parameters (the photoisomers absorption spectra and the quantum yields of partial photoreactions) that allowed development of adequate mathematical model [14,15].
The kinetics of previtamin D photosynthesis can be described by the system of rate equations [14] and numerically calculated for any monochromatic or polychromatic UV light source with the intensity distribution I0 (l ). This also provides a means for calculation of the action spectrum of "Vitamin D" biodosimeter. In Fig.4 the calculated action spectrum is shown together with provitamin D absorption spectrum in relative units. From close correspondence of the two spectra it is evident that if in
weighted spectroradiometry we are to estimate "antirachitic" effectiveness of UV radiation we can lean upon the absorption spectrum of provitamin D.|
|
|
Fig.4. Comparison of calculated action spectrum of previtamin D photosynthesis (1), absorption spectrum Of provitamin D (2) and the CIE erythema action spectrum (3). |
When the action spectra of vitamin D synthesis is compared with the CIE erythema action spectrum [16] (Fig.4), it is apparent that the curves behaviour in the UVA spectral region differs dramatically.
3. Results and discussion
"Vitamin D" biodosimeter has been put to the laboratory and field tests within the framework of the EC Project "BIODOS". We investigated the validity of the model by simultaneously measuring artificial and solar UV radiation using the spectroradiometer and "Vitamin D" biodosimeter. Here we will show and briefly discuss some results.
Several experiments were performed using narrow band filters and calibration facility at the Belgium Institute of Space Aeronomy and the required doses to get 10% of previtamin D have been determined (Table 1).
Table1.
|
Wavelength (nm) |
260 |
270 |
280 |
290 |
300 |
310 |
|
Dose (J/m2) |
111 |
85.3 |
80 |
182.7 |
1473 |
20850 |
|
MED (200 J/m2) |
0.56 |
0.43 |
0.4 |
0.91 |
7.37 |
104.3 |
Laboratory tests with artificial UV sources were conducted at the Institute of Biophysics, Budapest (1996). Ethanol solutions of 7-DHC were irradiated in quartz cuvettes by the three UV lamps: (1) FS20, (2) TL01, and (3) Solar Simulator. The spectral irradiance of the lamps at the cuvette positions has been measured by spectroradiometer, and corresponding data are presented in Fig.5a) together with the provitamin D absorption spectrum. Figure 5b) shows associating concentrational kinetics of provitamin D decay and previtamin D accumulation. As is easy to see, not only the photoreaction rate differs essentially for the three lamps but maximum achievable concentrations of previtamin D are distinguishable as well. This last-named parameter affords spectral selectivity of the method (exceptional sensitivity to the spectral composition of UV radiation) [2].
Additional test has been done at the UMIST (Manchester) with the standard lamp BBH4 (1000W FEL, Osram Sylvania Ltd) which is commonly used for spectroradiometer calibration. Cuvette with ethanol solution of 7-DHC was irradiated at the standard distance of 50 cm. The absorption spectra were recorded by a Perkin-Elmer Lambda 15 UV/VIS spectrophotometer before and after several exposures, and were further processed by computer to derive the photoisomer concentrations. The photoreaction kinetics was calculated using the spectral data of the BBH4 lamp as input for the system of differential equations [14,15]. Close correspondence of the experimental and calculated data has been found (Fig.6a).
|
. |
|
|
Figure 5. a) UV radiation spectra of the three lamps together with provitamin D absorption spectrum; b) Comparison of experimental (symbols) and calculated (lines) kinetics of provitamin D decay and previtamin D accumulation upon irradiation by the three lamps. |
|
The same experiment was performed for a 25cm distance between the lamp and the cuvette to increase the doses without requiring excessively long exposure times. It is hardly surprising that a doubling of the distance between the lamp and cuvette led to a four fold increase of the exposure time to get the same concentration of previtamin D (Fig.6b)
|
|
|
|
Fig.6 Experimental (symbols) and calculated (dotted lines) kinetics of previtamin D photosynthesis upon Irradiation by the BBH4 lamp: a) 50 cm distance, b) 25 cm distance (for calculation the lamp intensity Was increased by factor 4) |
|
First measurement of solar radiation with "Vitamin D" biodosimeter has been performed during the field intercomparison in Thessaloniki in July, 1997. The cuvettes were exposed during a day and the absorption spectra were recorded before exposure and every hour (30 min around noon) during the day. The photoreaction kinetics was calculated using the time-averaged spectroradiometer data. The corresponding experimental and calculated kinetic are presented in Fig.7 where the non-exponential character of provitamin D decay is readily apparent. The method reproducibility is demonstrated in Fig.7 by good coincidence of the results for the two cuvettes exposed in the same conditions.
In June, 1998 the solar UV measurement was conducted in Manchester. Seven identical cuvettes with ethanol solution of 7-DHC were exposed in the horizontal position close to the spectroradiometer diffuser. The absorption spectra were recorded every hour. During the time when the cuvettes were exposed, the solar spectrum was recorded every ten minutes by the spectroradiometer, a Bentham DTM300 double monochromator. Then the data obtained with the spectroradiometer were averaged over every hour and used as input for the mathematical model of the "Vitamin D" dosimeter.
|
|
|
Figure 7. Drawing of provitamin D decay and provitamin D accumulation during daily exposure to solar radiation: symbols – experimental data (two cuvettes in the same position), lines – calculated data. |
Additionally, the minimal erythema doses (MED) were calculated from the spectroradiometer data for every one hour exposure (1 MED = 200 J/m2), and good correlation of experimentally measured concentrations of previtamin D with calculated MED was found (Fig.8). ). However, calculated concentrations of previtamin D were permanently lower than the experimentally measured. Two factors were suggested to be responsible for the discrepancy. The first one is the geometrical factor. The diffuser of the spectroradiometer has an angular response that is a reasonable approximation to a true cosine response, while this is far from the case for the "Vitamin D" biodosimeter. This difference in angular acceptance of the two devices is especially important when the zenith angle is large (high latitudes, early morning and evening time). A second, related, cause lies in the mathematical model where direct irradiation of the absorbing layer is suggested. This condition is fulfilled in the laboratory tests under lamp irradiation, but obviously was not met for solar irradiation in Manchester (contrary to clear midsummer day in Greece where more radiation was in the direct beam, and entered the cuvette at an angle closer to normal).
|
|
|
Figure 8. Relation between accumulation of previtamin D and MED per 1 hour (Linear fit (y = Bx): B = 2.213) |
4. Conclusion
The results obtained confirm the ability of the "Vitamin D" biodosimeter to provide a reliable measure of the antirachitic UV solar radiation, although further work is needed to address the angular response of the dosimeters, and the model assumption of normal incidence. The method is sensitive enough to enable the UV dose measurement with a time resolution of 0.5-1 hour.
The availability of a mathematical model for previtamin D photosynthesis (from measured or modelled spectra) will allow prediction of profiles of antirachitic solar UV radiation over the globe.
Further studies are required to establish the correspondence between the in vitro "Vitamin D" dosimeter readings and previtamin D accumulation in vivo.
5. Acknowledgment.
The current research of the author is supported by grant from the EC (ENV4-CT95-0044) and the Fellowship from the British Royal Society. The author is grateful to Dr. A. Webb, Dr. D. Bolsee and Dr. O. Galkin for cooperation in this investigation, and to Dr. G. Horneck, Dr. D. Gillotay, Dr. A. Bais and Prof. G. Ronto for helpful discussions.
References
1. A.R. Webb, Measuring UV radiation: a discussion of dosimeter properties, uses and limitations, J.
Photochem. Photobiol. B: Biology, 31 (1995) 9-13.
2. I.P.Terenetskaya, Provitamin D photoisomerization as possible UVB monitor: kinetic study using
tunable dye laser", SPIE Proceedings, 2134B "Ultraviolet Radiation Hazards", (1994) 135-140.
3. A.C. Mayar and A.W. Norman, Vitamin D, in: Encyclopedia of Human Biology, Academic Press, Inc.
v.7 (1991) 859-871.
4. W.E.Stumpf and T.H.Privette, Light, vitamin D and psychiatry, Psychopharmacology, 97 (1989) 285-
294.
5. M.F.Holick, Vitamin D3 and sunlight: an intimate beneficial relationship, in M.F. Holick et al. (eds.),
Biologic Effects of Light, , W. de Gruyter, Berlin, New York, 1992, pp. 11-33.
6. M.F.Holick, S.A.Holick and R.L.Guillard, 1982. In: Comparative Endocrinology and Calcium
Regulation, (C. Oguro and P.K.T. Pang, eds.) Sci. Soc. Press, Tokyo, 85-91.
7. A.R.Webb, L.W.Kline and M.F.Holick, Influence of season and latitude on the cutaneous synthesis of
vitamin D3: exposure to winter sunlight in Boston and Edmonton will not promote vitamin D3 in
human skin, J. Clin. Endocrinol. Metabol., 67 (1988) 373-378.
8. I. Galindo, S. Frenk and H. Bravo, Ultraviolet irradiance over Mexico city, J. Air & Water Manage
Assoc. 45 (1995) 886-892.
9. a) F.C. Garland, C.F. Garland, et al. The positive association of breast cancer mortality with low sunlight
levels and high air pollution levels in major U.S. cities, in M.F. Holick et al. (eds.), Biologic Effects of
Light, , W. de Gruyter, Berlin, New York, 1992, pp.34-38.
b) C.F. Garland, F.C. Garland, et al., Sunlight, vitamin D, and mortality from breast and colorectal
cancer in Italy, ibid. pp.39-43.
c) E.C. Gorham, F.C. Garland and C.F. Garland, Geographic variation in breast cancer incidence and
sunlight in the USSR: the possible protective effects of vitamin D3 and 25-hydroxyvitamin D3, ibid.
pp.68-72.
10. I.P. Terenetskaya, N.A. Bogoslovsky, et al. Routes to optimization of previtamin D photosynthesis
using irradiation by a sunlamp, Pharm. Chem. J., 28 (1994) 589-596.
11. K.H. Hanewald, M.P. Rappoldt and J.R. Roborgh, The antirachitic activity of previtamin D3, Rec.
Trav.Chim. 80 (1961) 1003-1014.
12. E. Havinga, Vitamin D, example and challenge, Experentia, 29 (1973) 1181-93.
13. H.J.C. Jacobs and E. Havinga, Photochemistry of vitamin D and its isomers and of simple trienes, in:
J.N. Pitts et al.(eds.), Advances in photochemistry, Wiley, New York, 11 (1979) 305-373.
14. S.I. Gundorov, V.A. Davydenko, I.P. Terenetskaya, et al., Study of the kinetics and quantum
efficiencies in the photochemistry of provitamin D using a tunable laser, Sov. J. Quantum Electron.,
21 (1991) 339-343.
15. I.P. Terenetskaya, O.N. Galkin and N.A. Bogoslovsky, Irreversible reactions in the photochemistry of
provitamin D under optically dense layer conditions, Pharm.Chem. J., 27 (1993) 282-288.
16. Mc. Kinlay and A.F. Diffey, A reference action spectrum for ultraviolet induced erythema in human
skin, CIE J., 66 (1987) 17-22.