Induction of a mycosporine-like amino acid (MAA) in the rice field cyanobacterium Anabaena sp. by UV irradiation
Rajeshwar P. Sinha, Manfred Klisch, Donat-P. Häder*
Institut für Botanik und Pharmazeutische Biologie, Friedrich-Alexander-Universität, Staudtstr. 5, D-91058 Erlangen, Germany
*Corresponding author: Tel.: +49 9131 8528216; fax: +49 9131 8528215; e-mail:
dphaeder@biologie.uni-erlangen.de
Abstract
An ultraviolet-absorbing mycosporine-like amino acid (MAA) was detected in a filamentous and heterocystous cyanobacterium, Anabaena sp., isolated from a rice paddy field near Varanasi, India. The MAA was isolated and purified by HPLC. Only one MAA was detected with a retention time of around 2.8 min and an absorption maximum at 334 nm. The MAA was identified as shinorine, a bisubstituted MAA containing both glycine and serine groups. There was an increase in the amount of MAA when the cultures were exposed to PAR + UV in comparison to the cultures exposed to PAR only. This shows that UV stress induces the synthesis of MAA in this cyanobacterium and thus may protect the organism against deleterious high solar radiation particularly during summer seasons in the tropics.
Keywords: Anabaena; HPLC; MAA; PAR; Spectroscopy; UV
Abbreviations: HPLC, high performance liquid chromatography; MAA, mycosporine-like amino acid; PAR, photosynthetically active radiation; UV, ultraviolet.
Introduction
The nitrogen-fixing cyanobacteria form a prominent component of microbial populations in wetland soils, especially in rice paddy fields, where they significantly contribute to fertility as a natural biofertilizer [35 and references therein]. Like all photoautotrophs, members of cyanobacteria depend on solar radiation as the primary source of energy in their natural environment. The potential threat to these cyanobacterial communities is the continuous depletion of the stratospheric ozone layer, as a result of anthropogenically released atmospheric pollutants such as chlorofluorocarbons (CFCs) and the consequent increase in solar ultraviolet-B (UV-B; 280-315 nm) radiation reaching the Earth's surface [2; 9; 23; 26]. Ozone depletion is expected to increase and spread to a broader range of latitudes well into the next century [14; 38] and predicted to recover until 2065 [27]. Because of its high energy, UV-B easily destroys proteins, DNA and other biologically relevant molecules [4; 18; 19; 35; 40; 41].
Since photosynthetic organisms are simultaneously exposed to visible and UV radiation in their natural habitat, they tend to develop mechanisms counteracting the damaging effects of UV. Besides repair of UV-induced damage of DNA by photoreactivation and excision repair [5; 24] and accumulation of carotenoids and detoxifying enzymes or radical quenchers and antioxidants that provide protection by scavenging harmful radicals or oxygen species [29; 30], an important mechanism to prevent UV-induced photodamage is the synthesis of UV absorbing compounds.
Phenylpropanoids, mainly flavonoid derivatives, located in the epidermis have been reported to protect higher plants from absorbing harmful UV radiation [37; 25]. Mycosporine-like amino acids (MAAs) and the sheath pigment, scytonemin, are thought to accomplish a similar function in cyanobacteria [13; 16; 17; 20; 42]. MAAs are water soluble substances characterized by a cyclohexenone or cyclohexenimine chromophore conjugated with the nitrogen substituent of an amino acid or its imino alcohol, having absorption maxima ranging from 310 to 360 nm [31]. MAAs have been identified in a number of taxonomically diverse organisms such as fungi [15], marine heterotrophic bacteria [1], cyanobacteria [17; 21], eukaryotic algae [7; 20; 22], marine invertebrates [20; 34], fish [12] and a wide variety of other marine organisms [6; 10; 11; 20; 28].
To date, most of the cyanobacteria reported to possess MAAs have been isolated from extreme habitats such as desert rocks, hot springs, tree barks etc. Since rice field cyanobacteria are also exposed to high irradiances particularly during hot summer seasons in the tropics, we tested Anabaena sp. for the presence of MAAs and the inducibility of MAA synthesis by UV radiation. To our knowledge this is the first report of the existence of MAA in a rice field cyanobacterium.
Materials and methods
The organism
The test organism Anabaena sp., a filamentous and heterocystous cyanobacterium, was isolated from a rice paddy field near Varanasi, India as described earlier [36]. Cultures were routinely grown in an autoclaved liquid medium [33] in Erlenmeyer flasks filled to 40 % of their nominal volume and placed in a culture room at a temperature of 20 + 2 °C and white fluorescent light at 12 + 2 W m-2. Unless otherwise stated, all experiments were performed with log phase cultures having an initial dry weight of approximately 0.1 mg ml-1.
PAR and UV irradiation source
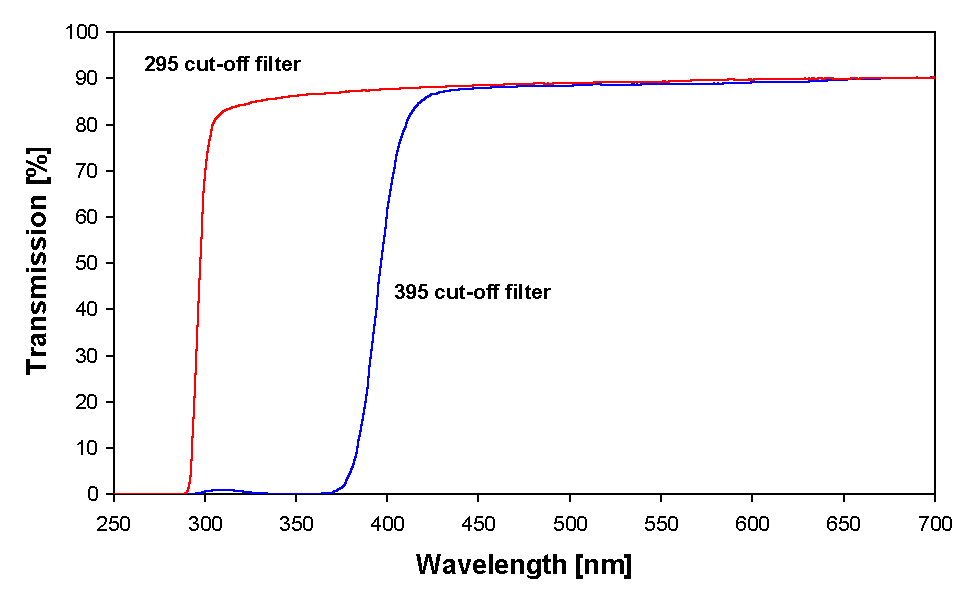
Open glass Petri dishes (75 mm in diameter) containing cultures of Anabaena sp. were placed on a rotary shaker (LQ-370 Electronic Born, Gerätebau, Gladenbach, Germany) to avoid self-shading. The cultures were irradiated for two days, simultaneously under artificial radiation of ultraviolet-B (UV-B; 280–315 nm), ultraviolet-A (UV-A; 315–400 nm) and fluorescent light (PAR; 400–700 nm), in the following referred to as PAR + UV. A 395 nm cut off filter foil (Ultraphan, UV Opak, Digefra, Munich, Germany) was placed over the Petri dish in the PAR only treatments to produce only the PAR waveband. In the PAR + UV treatments UV-C irradiation was eliminated by a 295 nm cut-off filter foil (Ultraphan, Digefra, Munich, Germany). Transmission spectra of the filter foils used are given in Fig. 1.

Figure 1. Transmission spectra of the filter foils used in the experiments.
UV-B irradiation was provided by a Philips Ultraviolet-B TL 40 W/12 (Holland) fluorescence tube with its main output at 312 nm. The irradiation was adjusted to about 1.0 W m-2. UV-A irradiation was provided by a UV-A-340 fluorescence tube (Q-Panel Co., Cleveland, Ohio, USA) with its main output at 340 nm. The irradiation was fixed at about 1.0 W m-2. The source of visible (PAR) light was OSRAM L 36 W/32 Lumilux de luxe warm white and Radium NL 36 W/26 Universal white (Germany), the irradiance of which was fixed at about 12 W m-2. The irradiances of the light sources were measured with a double monochromator spectroradiometer (OL 754, Optronic Laboratories, Orlando, FL, USA).
Extraction and partial purification of mycosporine-like amino acid (MAA)
Cells were harvested by centrifugation (J2-21M/E) using a JA 20 rotor (Beckman Instruments, Palo Alto, CA, USA) at 1500 x g for 10 min at room temperature. Samples were extracted in 5 ml of 20 % (v/v) aqueous methanol (HPLC grade) by incubating at 45 °C for 2.5 h. After centrifugation (5000 x g; GP centrifuge, Beckman, Palo Alto, USA) the supernatant was lyophilized (Lyovac GT 2, Leybold, Köln, Germany) and redissolved in 2 ml 100 % methanol, vortexed for 2-3 min (L46, GLW, Würzburg, Germany) and centrifuged at 10 000 x g for 10 min (Sigma2-MK, Sigma Laborzentrifugen GmbH, Osterode, Germany). Thereafter a 1.5 ml aliquot of the supernatant was evaporated to dryness at 45 °C and the extract redissolved in 1.5 ml of 0.2 % acetic acid. The samples were filtered through 0.2 µm pore-sized microcentrifuge filters (Mikro-Spin Zentrifugenfilter, Roth, Karlsruhe, Germany) in order to partially purify the MAA. This partially purified MAA was subjected to HPLC analysis.
High performance liquid chromatography (HPLC)
Further analysis and purification of partially purified MAA was performed using a HPLC system (Merck Hitachi; Interface D-7000, UV-Detector L-7400, Pump L-7100, Darmstadt, Germany) equipped with a LiCrospher RP 18 column and guard (5 µm packing; 250 4 mm I.D.). The sample was injected with a Hamilton syringe (Switzerland) into the HPLC column through a Rheodyne (USA) injection valve equipped with a 20 µl sample loop. The wavelength for detection was 330 nm at a flow rate of 1.0 ml min-1 and a mobile phase of 0.2 % acetic acid. Identification of the MAA was done by comparing the absorption spectra and retention times with several standards kindly provided by Dr. Ulf Karsten, Alfred-Wegener-Institut, Bremerhaven, Germany.
Absorption and fluorescence spectroscopy
Absorption spectra of samples were measured in a single beam spectrophotometer (DU 70, Beckman, Palo Alto, USA). The raw spectra were transferred to a microcomputer and treated mathematically and statistically using the software provided by the manufacturer (Beckman, Palo Alto, USA). Fluorescence emission spectra were recorded with a spectrofluorometer (RF-5000, Shimadzu, Kyoto, Japan) at room temperature. Further evaluation was performed on a microcomputer using the RF-PC software developed by Shimadzu, Japan.
Results
MAAs were partially purified from both samples as described earlier. Absorption spectra showing various steps of purification are illustrated in Figures 2A and B. Extraction of samples with 20 % methanol at 45 °C for 2.5 h resulted in a prominent peak at 334 nm (MAA) in all samples. There was an increase in the absorbance around 334 nm in the samples irradiated with PAR + UV (Figs. 2A and B) and also, but to a much lesser extent, in the samples that received only PAR. In addition to MAA, small amounts of photosynthetic pigments were also extracted by this procedure (Fig. 2A). MAA samples were further treated with 100 % methanol in order to remove proteins and salts and finally with 0.2 % acetic acid to remove unpolar photosynthetic pigments. The resultant partially purified MAAs had an absorption maximum at 334 nm with a small tail at around 400 nm (Fig. 2B).
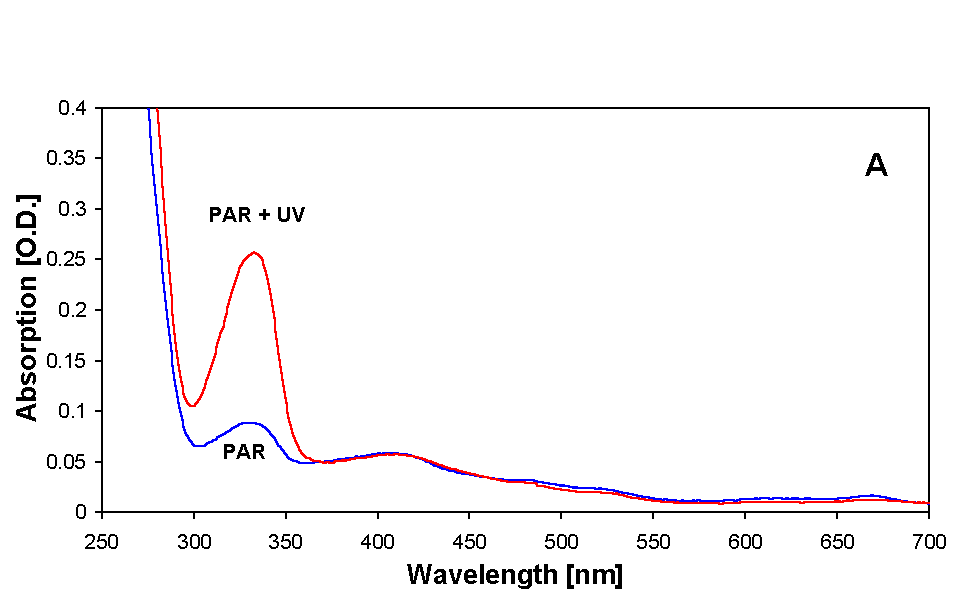
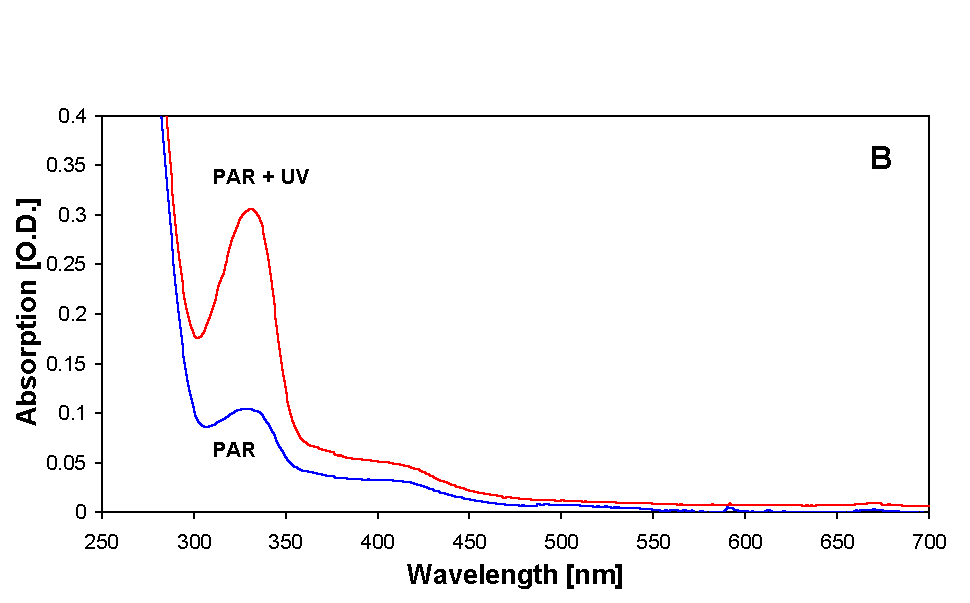
Figure 2. Absorption spectra showing MAAs of both PAR + UV and only PAR irradiated samples of Anabaena sp. after various steps of purification. (A) initial extract in 20 % methanol, (B) sample prepared for HPLC injection in 0.2 % acetic acid. For details see text.
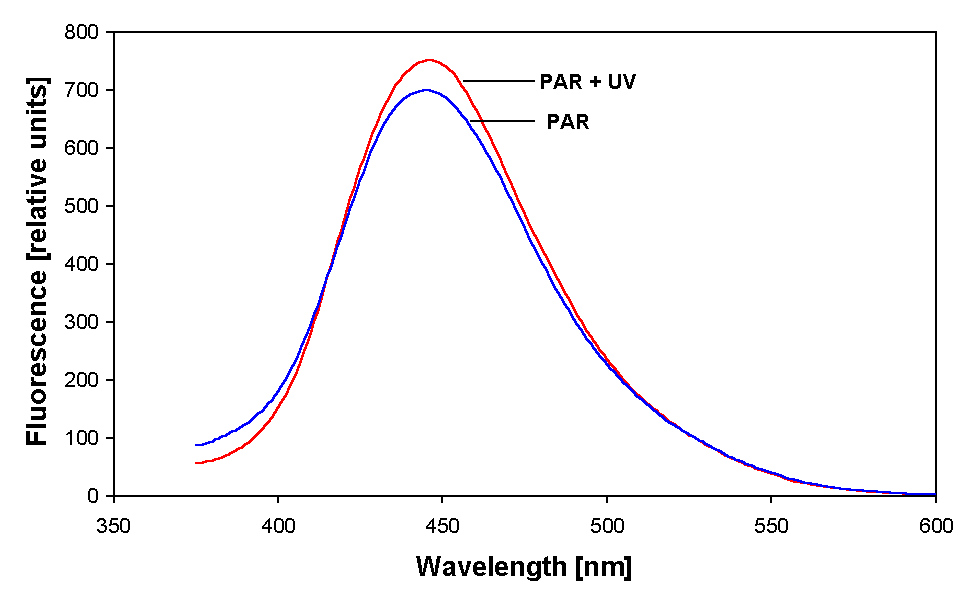
Figure 3. Fluorescence emission spectra of partially purified MAA of Anabaena sp. from both PAR + UV and only PAR irradiated samples.
Fluorescence excitation of partially purified MAAs at 334 nm resulted in an emission at around 436 nm in both PAR + UV and only PAR irradiated samples (Fig. 3). MAA in this cyanobacterium seems to be highly stable against UV and heat stress since its absorption properties were unaffected even after a UV and heat treatment as high as 3 W m-2 and 75 °C, respectively, for 6 – 8 h (data not shown).
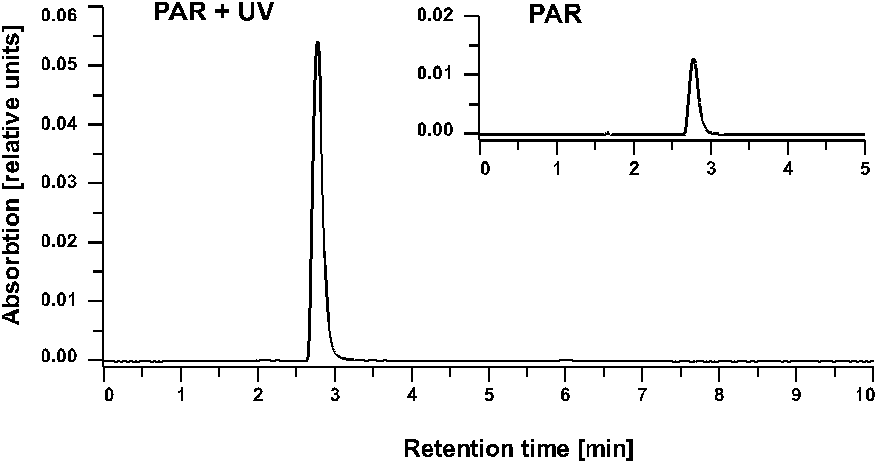
Figure 4. HPLC chromatograms of PAR + UV and only PAR irradiated samples of Anabaena sp. after 24 h irradiation time. LiCrospher RP 18 column and guard; mobile phase 0.2 % acetic acid; flow rate 1.0 ml min-1; detection by absorbance at 330 nm.
Further analysis and purification of the partially purified MAAs was done by HPLC. Only one prominent peak of MAA with a retention time of 2.8 (Fig. 4) and an absorbance maximum at 334 nm (Fig. 5) was recorded in all samples. By changing the eluant from 0.2 % to 0.02 % acetic acid the retention time was shifted to 2.1 min.
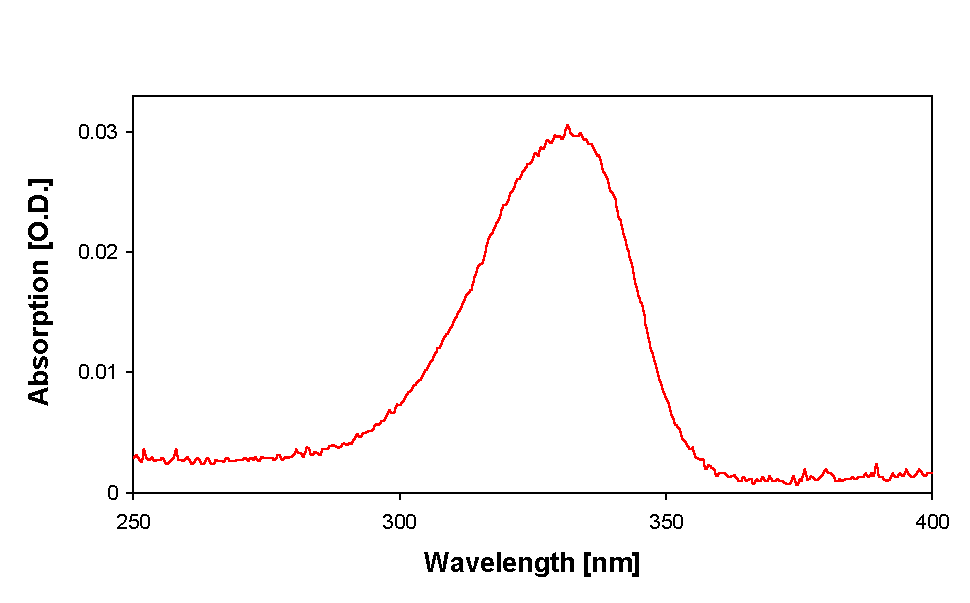
Figure 5. Absorption spectrum of purified MAA of Anabaena sp. as separated by HPLC.
HPLC analysis also revealed that there was an increase in the MAA content in the samples irradiated with PAR + UV in comparison to those irradiated with PAR only. Induction of MAA synthesis occurred within 24 h as indicated by the increase in peak area (Fig. 6) of the peak at 2.8 minutes. MAA was identified by comparison of wavelength ratios with standards as shinorine, a bisubstituted MAA containing both glycine and serine groups (Garcia-Pichel, personal communication).
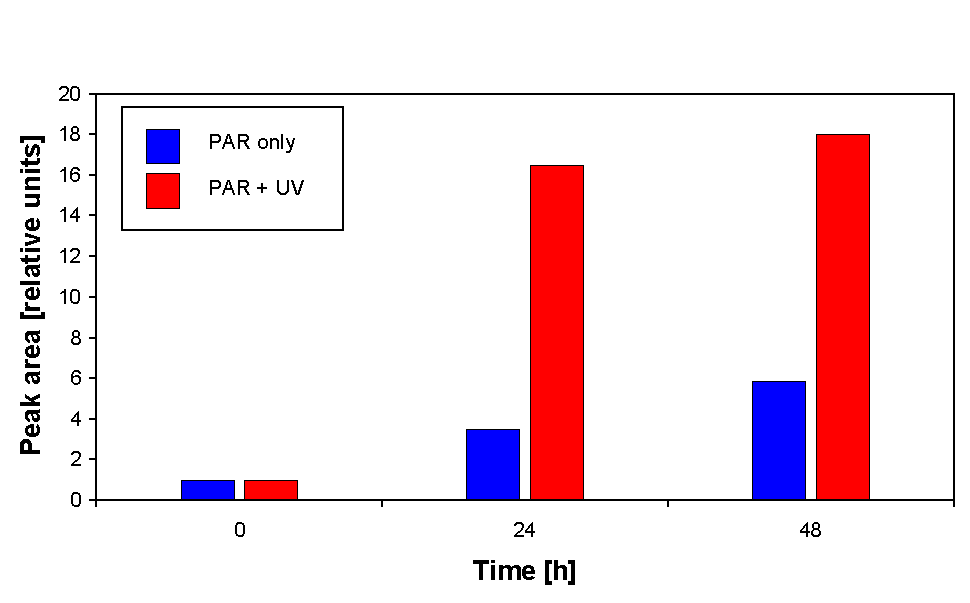
Figure 6. Relative increase of HPLC peak area during 48 h of irradiation. The peak area of the 0 h sample is set to 1.
Discussion
A number of organisms isolated from freshwater, marine or terrestrial habitats contain MAAs [10; 17; 20; 21]. Some of them have been identified but most still have to be characterized chemically. MAAs such as asterina-330, mycosporine-gly, porphyra-334, and shinorine are common in diverse types of organisms. Most of the cyanobacteria reported to contain MAAs have been identified from terrestrial habitats. Here we report the occurrence of MAA in a rice field cyanobacterium Anabaena sp. of Indian origin.
The occurrence of high concentrations of MAAs exposed to high levels of solar radiation has been described to provide protection as a UV-absorbing sunscreen [11; 22], but there is no conclusive evidence for the exclusive role of MAAs as sunscreen, and it is possible that they play more than one role in the cellular metabolism of all or some organisms [8]. It has been reported that MAAs may act as antioxidants to prevent cellular damage resulting from UV-induced production of active oxygen species [11]. Synthesis and excretion of MAA have been reported to be stimulated by UV-irradiation in a dinoflagellate, Lingulodinium polyedra [39] and MAAs were found to increase in response to PAR and UV irradiation in a red alga Chondrus crispus [22]. In many organisms MAA accumulation is strongly stimulated by UV-A [42] which has an about 50 times higher irradiance in natural solar radiation than UV-B.
The MAAs in Nostoc commune have been reported to be located extracellularly and linked to oligosaccharides in the sheath [3]. These glycosylated MAAs represent perhaps the only known example of MAAs that are actively excreted and accumulated extracellularly and therefore act as a true screen [13]. But intracellular MAAs are also effective protectants against UV-induced damage. Studies with other cyanobacteria have shown that MAAs prevent 3 out of 10 photons from hitting cytoplasmic targets. Cells with high concentrations of MAAs are approximately 25 % more resistant to UV radiation centered at 320 nm than those with no or low concentrations [17]. However there may be physiological limitations to the accumulation of osmotically active compounds such as MAAs within the cell, and it seems probable that the maximal specific content of MAAs in the cell is regulated by osmotic mechanisms which is reflected by the fact that field populations of halotolerant cyanobacteria contain unusually high concentration of MAAs [32].
While in the present study we did not attempt to chemically characterize the MAA, its spectral and other characteristics are comparable to those of shinorine. The fluorescence emission of partially purified MAA from Anabaena is at a wavelength overlapping with chlorophyll absorption, so that it is possible that the energy is being trapped by chlorophyll for subsequent transfer into the photosynthetic pathway.
The studied cyanobacterium Anabaena sp. is exposed to high levels of solar radiation particularly during the summer season in tropical rice growing countries. Regardless of the question whether the UV protective properties of MAAs are a primary or secondary function or whether they are synthesized or accumulated due to UV irradiation, the presence of these compounds in an organism may provide protection to the internal organelles and components from the full impact of deleterious UV radiation [20]. It is evident from the present investigation that the organisms are able to raise MAA content in response to UV stress within 24 h and thus may be able to adapt to day to day fluctuations in UV irradiation.
Acknowledgements
The help from F. Garcia-Pichel (MPI for Marine Microbiology, Bremen, Germany) with the identification of MAA is gratefully acknowledged. This work was financially supported by the C. S. I. R. (9/13(795)/96-EMR-I(RK)213108), New Delhi, India to R. P. Sinha and by European Union (DGXII, Environment programme, ENV4-CT97-0580) to D.-P. Häder. We thank M. Schuster for excellent technical assistance.
References
[1] Arai, T., Nishijima, M., Adachi, K., Sano, H., 1992. Isolation and structure of a UV absorbing substance from the marine bacterium Micrococcus sp. AK-334. MBI Report. Marine Biotechnology Institute, 2-35-10 Hongo, Bunkyo-ku, Tokyo, Japan. 113, 88-94.
[2] Blumthaler, M., Ambach, W., 1990. Indication of increasing solar ultraviolet-B radiation flux in alpine regions. Science 248, 206-208.
[3] Böhm, G.A., Pfleiderer, W., Böger, P., Scherer, S., 1995. Structure of a novel oligosaccharide-mycosporine-amino acid ultraviolet A/B sunscreen pigment from the terrestrial cyanobacterium Nostoc commune. J. Biol. Chem. 270, 8536-8539.
[4] Bothwell, M.L., Sherbot, D.M.J., Pollock, C.M., 1994. Ecosystem response to solar ultraviolet-B radiation: influence of trophic-level interactions. Science 265, 97-100.
[5] Britt, A.B., 1995. Repair of DNA damage induced by ultraviolet radiation. Plant Physiol. 108, 891-896.
[6] Carefoot, T.H., Harris, M., Taylor, B.E., Donovan, D., Karentz, D., 1998. Mycosporine-like amino acids: possible UV protection in eggs of the sea hare Aplysia dactylomela. Mar. Biol. 130, 389-396.
[7] Carreto, J.I., Carignan, M.O., Daleo, G., De Marco, S.G., 1990. Occurrence of mycosporine-like amino acids in the red tide dinoflagellate Alexandrium excavatum: UV-protective compounds? J. Plankton Res. 12, 909-921.
[8] Castenholz, R.W., 1997. Multiple strategies for UV tolerance in cyanobacteria. The Spectrum 10, 10-16.
[9] Crutzen, P.J., 1992. Ultraviolet on the increase. Nature 356, 104-105.
[10] Dunlap, W.C., Shick, J.M., 1998. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. J. Phycol. 34, 418-430.
[11] Dunlap, W.C., Yamamoto, Y., 1995. Small-molecule antioxidants in marine organisms: antioxidant activity of mycosporine-glycine. Comp. Biochem. Physiol. 112, 105-114.
[12] Dunlap, W.C., Williams, D.M., Chalker, B.E., Banaszak, A.T., 1989. Biochemical photoadaptations in vision: UV-absorbing pigments in fish eye tissues. Com. Biochem. Physiol. 93, 601-607.
[13] Ehling-Schulz, M., Bilger, W., Scherer, S., 1997. UV-B-induced synthesis of photoprotective pigments and extracellular polysaccharides in the terrestrial cyanobacterium Nostoc commune. J. Bacteriol. 179, 1940-1945.
[14] Elkins, J.W., Thompson, T.M., Swanson, T.H., Butler, J.H., Hall, B.D., Cummings, S.O., Fisher, D.A., Raffo, A.G., 1993. Decrease in the growth rates of atmospheric chlorofluorocarbons 11 and 12. Nature 364, 780-783.
[15] Favre-Bonvin, J., Arpin, N., Brevard, C., 1976. Structure de la mycosporine (P 310). Can. J. Chem. 54, 1105-1113.
[16] Garcia-Pichel, F., Castenholz, R.W., 1991. Characterization and biological impliations of scytonemin, a cyanobacterial sheath pigments. J. Phycol. 27, 395-409.
[17] Garcia-Pichel, F., Wingard, C.E., Castenholz, R.W. 1993. Evidence regarding the UV sunscreen role of a mycosporine-like compound in the cyanobacterium Gloeocapsa sp. Appl. Environ. Microbiol. 59, 170-176.
[18] Häder, D.-P., Worrest, R.C., Kumar, H.D., Smith, R.C., 1995. Effects of increased solar ultraviolet radiation on aquatic ecosystems. Ambio 24, 174-180.
[19] Karentz, D., Cleaver, J.E., Mitchell, D.L., 1991a. Cell survival characteristics and molecular responses of Antarctic phytoplankton to ultraviolet-B radiation. J. Phycol. 27, 326-341.
[20] Karentz, D., McEuen, F.S., Land, M.C., Dunlap, W.C., 1991b. Survey of mycosporine-like amino acid compounds in Antarctic marine organism: potential protection from ultraviolet exposure. Mar. Biol. 108, 157-166.
[21] Karsten, U., Garcia-Pichel, F., 1996. Carotenoids and mycosporine-like amino acid compounds in members of the genus Microcoleus (Cyanobacteria): a chemosystematic study. Syst. Appl. Microbiol. 19, 285-294.
[22] Karsten, U., Franklin, L.A., Lüning, K., Wiencke, C., 1998. Natural ultraviolet radiation and photosynthetically active radiation induce formation of mycosporine-like amino acids in the marine macroalga Chondrus crispus (Rhodophyta). Planta 205, 257-262.
[23] Kerr, J.B., McElroy, C.T., 1993. Evidence for large upward trends of ultraviolet-B radiation linked to ozone depletion. Science 262, 1032-1034.
[24] Kim, S.-T., Sancar, A., 1995. Photorepair of nonadjacent pyrimidine dimers by DNA photolyase. Photochem. Photobiol. 61, 171-174.
[25] Kootstra, A., 1994. Protection from UV-B induced DNA damage by flavonoids. Plant Mol. Biol. 26, 771-774.
[26] Lubin, D., Jensen, E.H., 1995. Effects of clouds and stratospheric ozone depletion on ultraviolet radiation trends. Nature 377, 710-713.
[27] Madronich, S., McKenzie, R.L., Caldwell, M.M., Björn, L.O., 1995. Changes in ultraviolet radiation reaching the Earth`s surface. Ambio 24, 143-152.
[28] Mc Clintock, J.B., Karentz, D., 1997. Mycosporine-like amino acids in 38 species of subtidal marine organisms from McMurdo Sound, Antarctica. Antarc. Sci. 9, 392-398.
[29] Middleton, E.M., Teramura, A.H., 1993. The role of flavonol glycosides and carotenoids in protecting soybean from ultraviolet-B damage. Plant Physiol. 103, 741-752.
[30] Mittler, R., Tel-Or, E., 1991. Oxidative stress responses in the unicellular cyanobacterium Synechococcus PCC7942. Free Radical Res. Commun. 12, 845-850.
[31] Nakamura, H., Kobayashi, J., Hirata, Y., 1982. Separation of micosporine-like amino acids in marine organisms using reverse-phase high performance liquid chromatography. J. Chromatogr. 250, 113-118.
[32] Oren, A., 1997. Mycosporine-like amino acids as osmotic solutes in a community of halophilic cyanobacteria. Geomicrobiol. J. 14, 231-240.
[33] Safferman, R.S., Morris, M.E., 1964. Growth characteristics of the blue-green algal virus LPP-1. J. Bacteriol. 88, 771-775.
[34] Shick, J.M., Dunlap, W.C., Chalker, B.E., Banaszak, A.T., Rosenzweig, T.K., 1992. Survey of ultraviolet radiation absorbing mycosporine-like amino acids in organs of coral reef holothuroids. Mar. Ecol. Prog. Ser. 90, 139-148.
[35] Sinha, R.P., Häder, D.-P., 1996. Photobiology and ecophysiology of rice field cyanobacteria. Photochem. Photobiol. 64, 887-896.
[36] Sinha, R.P., Häder, D.-P., 1996. Response of a rice field cyanobacterium Anabaena sp. to physiological stressors. Env. Exp. Bot. 36, 147-155.
[37] Tevini, M., Braun, J., Fieser, G., 1991. The protective function of the epidermal layer of rye seedlings against ultraviolet-B radiation. Photochem. Photobiol. 53, 329-333.
[38] Toon, O.B., Turco, R.P., 1991. Polar stratospheric clouds and ozone depletion. Sci. Am. 264, 68-74.
[39] Vernet, M., Whitehead, K., 1996. Release of ultraviolet-absorbing compounds by the red-tide dinoflagellate Lingulodinium polyedra. Mar. Biol. 127, 35-44.
[40] Vincent, W.F., Roy, S., 1993. Solar ultraviolet-B radiation and aquatic primary production: damage, protection, and recovery. Environ. Rev. 1, 1-12.
[41] Williamson, C.E., 1995. What role does UVB radiation play in freshwater ecosystems? Limnol. Ocean. 40, 386-392.
[42] Xiong, F., Komenda, J., Kopecky, J., Nedbal, L., 1997. Strategies of ultraviolet-B protection in microscopic algae. Physiol. Plant. 100, 378-388.