A Laser Flash Photolysis Study of Remote Hydrogen Atom Abstraction from Phenols by Aromatic Ketone Triplets. Geometry Effects.
 |
Edward
C. Lathioor, William
J. Leigh,* and Michael J. St. Pierre Department of Chemistry, McMaster University, Hamilton, ON Canada 17th IUPAC Symposium on Photochemistry |
Questions or comments may be directed to Ed Lathioor.
INTRODUCTION
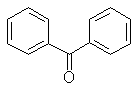
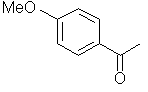
Photoreduction of aromatic ketone triplets commonly occurs via a radical-like hydrogen atom abstraction. In this mechanism, two main classes of ketone triplet distinguish themselves, n,p* and p,p*
 |
 |
|
| benzophenone | 4-methoxy acetophenone | |
| Triplet state: | n,p* | p,p* |
In general, the n,p* ketone reacts faster and more efficiently than the p,p* triplet. For some donors, the p,p* ketone triplet may not react at all.
The reactions of aromatic ketone triplets with phenols have been briefly studied in the past. No products can be easily isolated from preparative experiments even though the rate of quenching of the ketone triplet by phenol has been shown to be very fast in both polar and non-polar solvents.
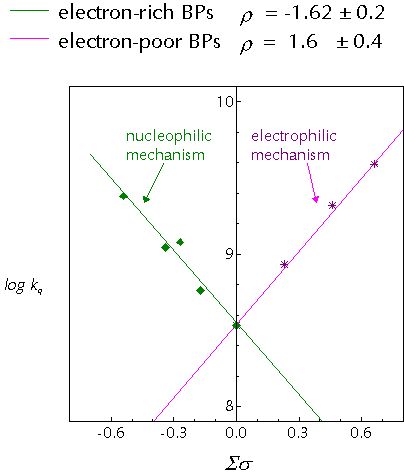
Recently, we published a study on the interactions of substituted benzophenones with para-cresol and discovered discontinuous Hammett behaviour:
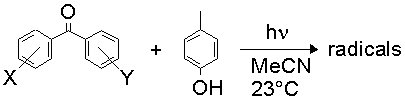
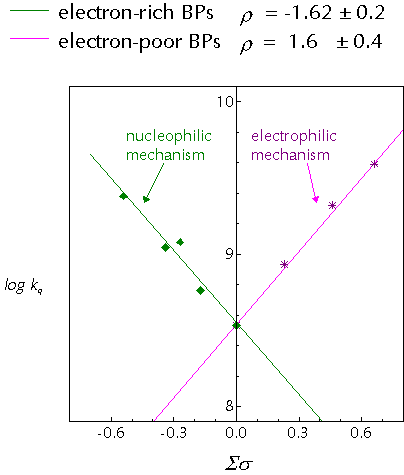
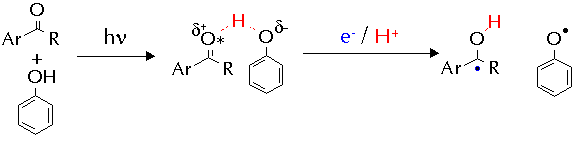
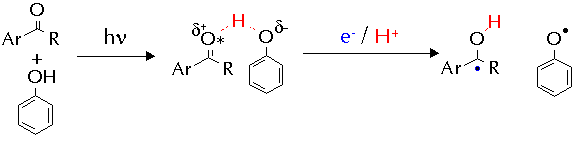
The two different mechanisms are thought to arrive from different behaviour of the ketone triplet. In the 'electrophilic' mechanism, the electron deficiency of the n,p* state is exacerbated by the action of strongly electron-withdrawing substituents and in response the triplet draws charge from the arene nucleus of the cresol. This partial charge transfer weakens the O-H bond in the phenol and the hydrogen atom transfer rate is enhanced.
The 'nucleophilic' mechanism represents novel behaviour for aromatic n,p* ketones, in which they behave as nucleophiles. A proposed mechanism to account for this involved hydrogen-bonding between the ketone triplet state and the phenol. The hydrogen-bond would speed formal hydrogen-atom transfer by allowing rapid electron/proton transfer to occur.
It is generally agreed that the p,p* states of aromatic ketones are more basic than the ground state. Thus, for benzophenones (lowest n,p* triplets) this type of behaviour points towards the involvement of the upper T2 (p,p*) state in the photochemistry. This is in agreement with the well known fact that the n,p*/p,p* splitting in substituted ketones decreases as the substituents become more electron donating.
RESULTS
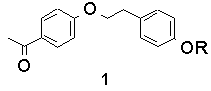
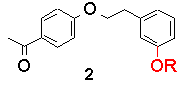
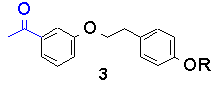
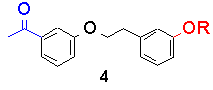
Recently, we looked at the intramolecular example, in bichromophoric molecules containing both ketone and phenol. The sensitivity of the transition state to geometry, if any, was to be tested by altering the geometry of linkage. The following table lists triplet lifetimes for substituted acetophenones and their anisyl-analogues:
Table 1. Absolute rate constants for quenching of para-methoxyacetophenone and meta-methoxyacetophenone triplets by para- and meta-cresol in deoxygenated acetonitrile solution*
Acetophenone Derivative |
kq (p-cresol) / 109 M-1 s-1 MeCN ** |
kq (m-cresol) / 109 M-1 s-1 MeCN *** |
4-methoxy |
1.24 ± 0.03 |
0.71 ± 0.02 |
3-methoxy |
1.32 ± 0.04 |
1.06 ± 0.05 |
* Measured by 337-nm laser flash photolysis of 0.01-0.03 M solutions of the ketones in deoxygenated acetonitrile
** Determined at 27°C
*** Determined at 29°C
Table 2. Triplet lifetimes for linked phenolic ketones in deoxygenated acetonitrile solution*
Ketone |
t* R = H |
t* R = Me |
|
14-ns |
2400-ns |
|
313-ns** |
2200-ns |
|
970-ns** |
2000-ns |
|
6600-ns** |
5500-ns |
* MeCN, room temperature, 0.8 to 5 x 10-4 M solutions, 248-nm laser excitation
** extrapolated lifetime at infinite dilution
The trend is that as the geometry is altered from a para/para relationship to a meta/meta one, the triplet lifetime greatly increases. However, the rates of bimolecular reaction between model ketones and phenols, i.e., p-methoxy acetophenone and p-cresol for 1-OH, DO NOT follow this trend and are all between 0.7 and 1.3 x109 M-1s-1.
DISCUSSION
Several possible explanations for this behaviour immediately come to mind. The first is that the geometrical changes distort the preferred geometry of the reaction and raise it's energy barrier(s), with the ultimate result that in the meta/meta case the molecule is so contorted that there is no hydrogen-atom abstraction. In the bimolecular case, there are no constraints and thus each set of molecules are free to adopt the lowest energy conformations and the rates of reaction are all high.
AM1 calculations performed on 1-OH and 4-OH indicate that there is little difference between the energies required to adopt sandwich-like quenching conformations in these two extremes. In fact, it should be even easier for 4-OH to adopt a geometry suitable for quenching than 1-OH.
Another possible explanation for the geometrical effect is electronic. Recall that in the mechanism given above, a hydrogen-bond is formed and then electron/proton transfer occurs. Although the hydrogen-bond can more or less equally form in the series, what about the electron transfer? The rate of such a process depends, amongst other things, on the amount of overlap between the initial and final electronic wave functions. If the overlap is good, then the electron transfer is allowed and should proceed at some near-maximum rate, and if the overlap is poor then the rate should be less than this maximum.
Below are depicted the calculated (AM1) orbital coefficients of the model ketones and phenols. The initial state is the cresol HOMO and the final state is the singly-occupied triplet state, or triplet SOMO.
- para case
Good overlap if stacked
4-methoxy acetophenone p-cresol
- meta case
Poor overlap if stacked
3-methoxy acetophenone m-cresol
Note that there is little electronic difference between the two phenols but a great difference between the 4- and 3-methoxy acetophenone.
This helps explain the results further. Compounds 1-OH and 2-OH, which only differ by the phenol attachment geometry, both have 'allowed' electron transfer by this model. The fact that 2-OH has a longer lifetime than 1-OH is due to the lesser intrinsic reactivity of m-cresol (see Table 1).
Similarly, 3-OH and 4-OH both have a 'forbidden' electron transfer. As a result, each are retarded by an order of magnitude over 1-OH and 2-OH.
CONCLUSION
The effect of geometry on the lifetimes in compounds 1-4 (R=OH) has been determined to originate from the orbital overlap between initial and final electronic states, not the ability of the different molecules to form correct quenching geometries.
ACKNOWLEDGEMENTS
We thank the Natural Sciences and Engineering Research Council of Canada for financial support.