Molecular Complexes Between Dimeric Forms of Porphyrin and Water
and Their Vibrational Dynamics
Alexander V. Udal’tsov* and Anatoly A. Churin
Faculty of Biology, Moscow State University, Moscow 119899 Russia
Singly and doubly protonated dimeric forms of meso-tetraphenylporphine were studied by absorption and resonance Raman spectroscopy in aqueous-organic solutions. The bands at 1000 and 1025 ± 2 cm–1 are revealed in the resonance Raman spectra of the porphyrin dimers. These bands characterize proton transitions of water molecules and hydroxonium ions associated with the porphyrin in dimeric forms. In the region of translational vibrations of water molecules, the spectra show a fine structure, whose bands produce doublets with the same interval of 25 ± 2 cm–1. In contrast, the Raman spectra of the corresponding aqueous-organic solutions exhibit a broad structureless band. Analysis of vibrational characteristics allowed one to advance a model structure of a dimeric complex with water for singly protonated dimer of meso-tetraphenylporphine.
Introduction
The problems related to the interaction of porphyrin with water molecules attract an interest from the viewpoint of studying the role of these pigments in light energy transformation in plants and algae [1-4]. Interaction of porphyrins with water also has a different aspect related to the fact that water is not only an initial component involved in the reduction-oxidation processes but also serves as an environment where all these processes are carried out. Therefore, an increased attention is focused on studying the effect of the state of water on the primary processes of charge separation in the photosynthetic reaction centers and model systems [5-7]. In connection with this, to obtain a necessary information, an important factor is related to an adequate selection of a model system, which is able to simulate not only interaction between porphyrins but also interaction of porphyrin with water. From this viewpoint, most advantageous candidates are the protonated dimeric systems of meso-tetraphenylporphine, which are formed in the aqueous-organic solutions as a result of competing between protonation reaction and spontaneous aggregation [8].
As compared with meso-tetra(p-aminophenyl)porphine, whose association is provided by amino groups in phenyl rings, the above dimers are produced via hydrophobic interactions, which are equilibrated by charge. This charge also prevents aggregation and precipitation of tetraphenylporphine. This behavior is responsible for specific features of intermolecular interactions in dimers, so in the case of tetraphenylporphine, only interactions between
p-orbitals of the porphyrin macrocycle are possible. Whereas, in the case of tetra(p-aminophenyl)porphine, in addition to the interactions between p-orbitals, donor-acceptor interactions are also allowed. These interactions involve nucleophilic amino groups that makes studying the interactions in dimers of porphyrin far more complicated.In this work, the singly and doubly protonated dimeric forms of meso-tetraphenylporphine, which are formed in aqueous-organic solutions, were investigated by absorption and resonance Raman spectroscopy.
Materials and Methods
Synthesis of meso-tetraphenylporphine was carried out according to the procedure described in [9]. Dioxane, tetrahydrofuran and other organic solvents were additionally purified by a conventional method [10]. For the preparation of aqueous-organic solutions, distilled or twice distilled water was used.
UV and visible spectra were recorded with a Specord M-40 spectrophotometer. Raman spectra of porphyrin solutions were obtained using the equipment described in [11]. Excitation was provided by an He-Cd laser into the Soret band (
lex = 441.6 nm). A spectral slit width of 4 cm–1 was used for registration. All measurements were carried out at 298K. All Raman spectra were recorded when concentration of porphyrin in the solution was lower than 4 ´ 10–5 M.Results
Figure 1 shows the absorption spectra of tetraphenylporphine in acetone (curve 1) and in aqueous solution of acetone in the presence of 0.4 N of hydrochloric acid (curve 2). In organic solvents porphyrin exists in its monomeric form, whereas in the presence of acid-containing aqueous-organic solutions, singly and doubly protonated forms of porphyrin dimers are produced [8]. The spectrum of the porphyrin (curve 2) with the maximum in the region of the Soret band at 437 nm corresponds to the doubly protonated dimer.
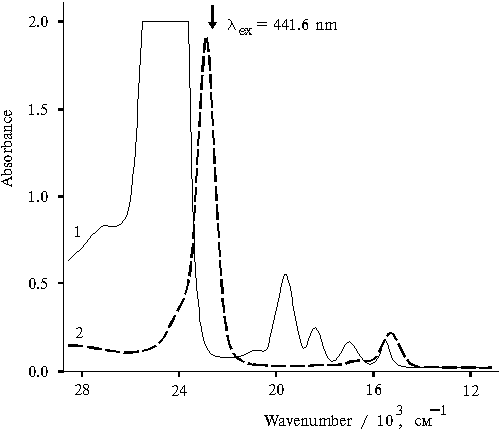
Fig. 1. Absorption spectra of meso-tetraphenylporphine in acetone, 1, and in 50% aqueous solution of acetone (v/v) in the presence of 0.4 N hydrochloric acid, 2.
The resonance Raman spectrum of the monomeric form of tetraphenylporphine in acetone shows the bands corresponding to the solvent and only strong bands corresponding to the porphyrin. The 1563 cm–1 band is the most intense among the bands of porphyrin (Fig. 2, curve 2). In this case, the bands at 208, 348, and 1014 cm–1, which are absent in the spectrum of the solvent (curve 1), are associated with certain virbational modes of solvent. As it will be shown below, the solvent molecules are involved in the solvate cover of porphyrin and interact with it. Also it is important to note that in the case of monomeric porphyrin, the bands corresponding to porphyrin have a low intensity as compared with those of the solvent.
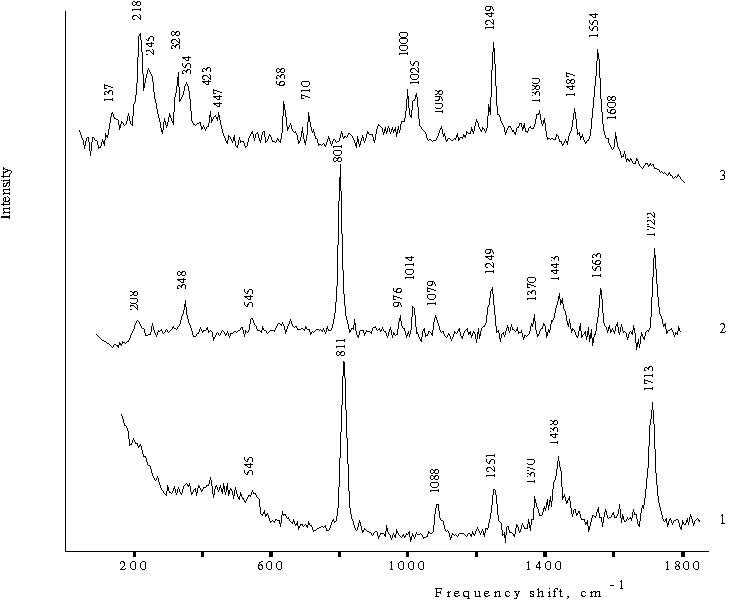
Fig. 2. Raman spectra of 35% acetone (v/v) in water in the presence of 0.4 N hydrochloric acid, 1; meso-tetraphenylporphine in acetone, 2, and in 50% aqueous solution of acetone (v/v) in the presence of 0.4 N hydrochloric acid, 3.
The resonance Raman spectrum of the doubly protonated form of tetraphenylporphine in the aqueous-organic solution (Fig. 2, curve 3) exhibit a set of doubled bands, which are located at a distance of 25 ± 2 cm–1 from each other. One doublet with maxima at 1000 and 1025 cm–1 is likely to correspond to the band at 1014 cm–1 in the spectrum of monomeric porphyrin (curve 2). Two other doublets are formed by highly intense bands and are located in the region of translational vibrations of water molecules at 218, 245, 328, and 354 cm–1. The 218 cm–1 band is one from the most intense spectral bands and is comparable to the band at 1554 cm–1 associated with planar vibrations of porphyrin. In the same region, the spectrum also shows two bands with low intensity at 423 and 447 cm-1, which are located at a distance of 25 ± 2 cm–1 from each other and form the similar doublet. Furthermore, the spectrum shows the two bands at 638 and 710 cm–1 with a medium intensity. Note that the spectrum (curve 3) shows no intense bands corresponding to solvent, which are usually observed at 811 and 801, 1438 and 1443, 1713 and 1722 cm–1 (curves 1 and 2 respectively).
Hence, according to this experimental evidence, acetone molecules do not participate in the formation of a solvate cover of doubly protonated porphyrin dimer in aqueous-acetone solution. At the same time, in the region of translational vibrations of water molecules, one may observe selective modes of water, which constitute a fine structure and characterize a vibrational water-porphyrin system.
Figure 3 shows the absorption spectra of singly and doubly protonated forms of tetraphenylporphine in aqueous-organic solutions of various compositions in the presence of 0.4 N hydrochloric acid. This evidence suggests that spectral pattern is primarily controlled by the specific features of interaction of dimeric forms of the porphyrin with solvent on the step of spontaneous formation of dimers. The ratio between three forms with maxima in the region of the Soret band at 403, 437, and 465 nm may be controlled by varying the composition of aqueous-organic solution. In the red spectral region, these three forms have absorption bands at 638, 654, and 694 nm, respectively. These forms are identified as one doubly protonated (
lmax = 437 nm) and two singly protonated dimers of porphyrin (lmax = 403 and 465 nm), which have different orientation of molecules in dimer [8].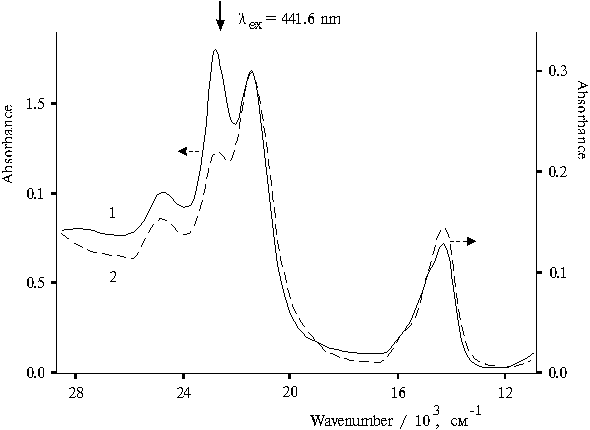
Fig. 3. Absorption spectra of meso-tetraphenylporphine in the presence of 0.4 N hydrochloric acid in water-acetone-dioxane solution (92.5 : 5 : 2.5), 1, and in water-glycerol-tetrahydrofuran solution (86.5 : 10 : 3.5), 2.
Figure 4 shows the resonance Raman spectra of the singly and doubly protonated forms of tetraphenylporphine in aqueous-organic solutions in the presence of 0.4 N hydrochloric acid. Both spectra show doublets with maxima at 1000 and 1025 cm–1 and fine structure in region of translational vibrations of water molecules with maxima at 122, 218, 242, 328, 355, and 422 cm–1 (curve 1). Almost the same set of frequencies is observed in the spectrum with different ratio between singly and doubly protonated dimeric forms of porphyrin (curve 2). However, in aqueous-organic solution containing glycerol, the corresponding spectrum of porphyrin dimers shows no bands at 422 and 844 cm–1. The doublet with maxima at 1000 and 1025 cm–1 does not change its shape and position of maxima in the spectra of doubly protonated dimer of porphyrin (Fig. 2, curve 3) and in the spectra of both types of dimers of singly and doubly protonated porphyrins (Fig. 4). Although, the 1025 cm–1 band is slightly decreased in the spectrum (curve 2). Hence, characteristics of this doublet are independent of variations in the composition of aqueous-organic solution.
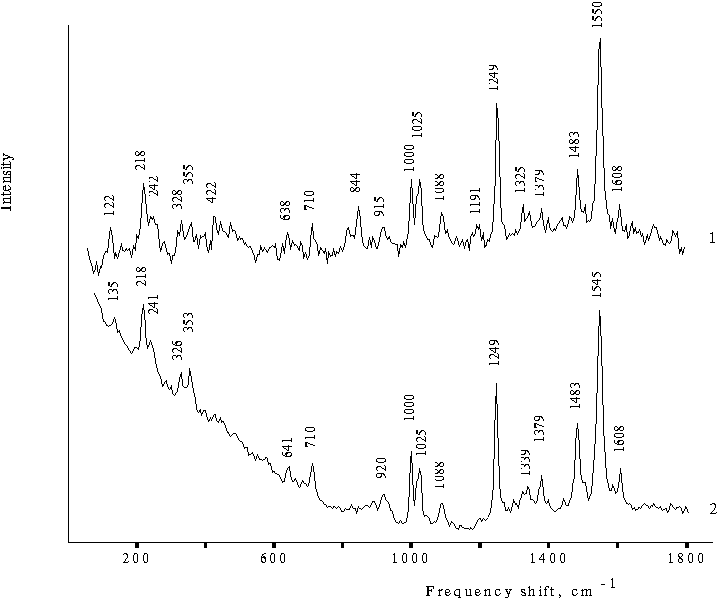
Fig. 4. Raman spectra of meso-tetraphenylporphine in the presence of 0.4 N hydrochloric acid in water-acetone-dioxane solution (92.5 : 5 : 2.5), 1, and in water-glycerol-tetrahydrofuran solution (86.5 : 10 : 3.5), 2.
In contrast, two doublets with highly intense bands in the region of translational vibrations of water molecules with maxima at 218 and 245 cm–1, 328 and 353 cm–1 (Fig. 2, curve 3) change their appearance when the solution also contains singly protonated forms of porphyrin dimers in addition to doubly protonated dimer. In this case, the intensities of the bands of the fine structure in the region of translational vibrations of water (Fig. 4) are much lower as compared with the corresponding bands of doubly protonated porphyrin dimer (Fig. 2, curve 3). The intensity of the most intense band involved in one of the doublets appears to be almost the same as the intensity of the 1000 cm–1 band (Fig. 4). The bands at 1550 and 1545 cm–1 are most intense in the spectra (curves 1 and 2, respectively). These spectra also show the band at 1608 cm–1, which is also seen in the spectra of doubly protonated dimer of porphyrin (Fig. 2, curve 3). According to the absorption spectra (Fig. 3), the ratios between the different dimeric forms of porphyrin in various solutions markedly differ from each other. However, the corresponding Raman spectra (Fig. 4) appear to be quite similar taking into account small difference.
In the case of porphyrin-free aqueous-organic solutions, the spectra show the bands, which are likely to be associated with the development of hydrogen bonds between water molecules and organic component. The Raman spectrum of aqueous glycerol solution shows a wide band at 1060-1130 cm–1 (Fig. 5, curve 1), whereas the spectrum of the three-component solution (water-tetrahydrofuran-glycerol) shows another band at 934 cm–1 (curve 2). In the case of porphyrin-free aqueous-organic solutions, the corresponding Raman spectra exhibit no fine structure in the region of translational vibrations of water as was observed for the solutions containing protonated dimeric forms of porphyrin. In contrast, a broad band with maximum at about 470 cm–1 and its half-width equaled to 145 cm–1 is observed (curve 1). A broad band in the region of translational vibrations of water is also seen in the Raman spectrum of water-acetone solutions (Fig. 2, curve 1). The spectrum of the same three-component solution but, in the presence of hydrochloric acid, shows another band at 905 cm–1 (Fig. 5, curve 3).
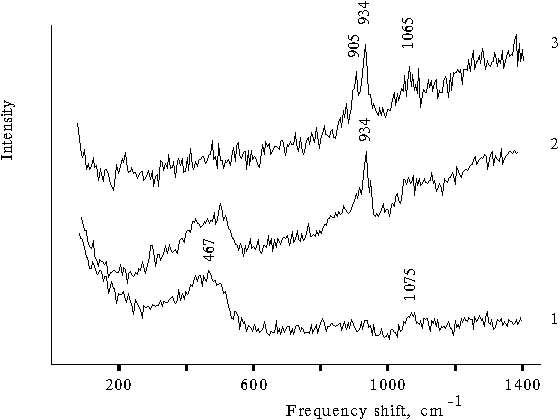
Fig. 5. Raman spectra of water-glycerol solution (90 : 10) in the presence of 0.4 N hydrochloric acid, 1; water-glycerol-tetrahydrofuran solution (1 : 1 : 1), 2, and water-glycerol-tetrahydrofuran solution (60 : 10 : 30) in the presence of 0.4 N hydrochloric acid, 3.
Hence, the Raman spectrum of porphyrin-free aqueous-organic solution also shows a similar doublet containing the bands located at a distance of 30 cm–1 from each other. As compared with the corresponding doublet in the Raman spectra of dimeric forms of porphyrin, this doublet is shifted to a low-frequency region by 90 - 95 cm–1. In the region of translational vibrations of water, the spectra of porphyrin-free aqueous-organic solutions show a broad band without any fine structure.
Discussion
The results presented above suggest that the Raman spectra of the singly and doubly protonated dimeric forms of the porphyrin reveal a fine structure in the region of translational vibrations of water, which characterizes the vibrational properties of water-porphyrin dimeric system. The presence of the fine structure in the spectrum is accompanied by the existence of the two closely located bands with medium intensities at 1000 and 1025 cm–1, which produce a typical doublet. In the presence of singly protonated dimers of tetraphenylporphine in the solution containing doubly protonated dimer, the fine structure in the region of translational vibrations of water changes, in particular the position of weak bands is some shifted. In this case, the intensity of the bands of the fine structure related to the 1000 cm–1 band, is about two-fold lower than that in the Raman spectrum of the doubly protonated dimer.
In the case of Raman spectrum of water, the broad band lower 1000 cm–1 is associated with librational vibrations of water [12]. The frequency lines of librational vibrations at about 1000 and 700 cm–1 are rather unusual and observed in the Raman spectra of ordinary and deuterated water in the solid state, respectively [13]. In the Raman spectrum of ice of their equimolar mixture, these frequencies are observed at 490 and 800 cm–1. Usually, these bands are considerably broadened as compared with the corresponding band in the Raman spectra of the protonated dimeric forms of porphyrin. The frequencies at 1000 and 700 cm–1 in the Raman spectra of ordinary and deuterated water were assigned to proton and deuterium transitions of hydrogen-bound water molecules [14]. These transitions may proceed synchronically in water clusters in liquid or solid state. In the similar region, the Raman spectrum of aqueous-organic solutions shows a band at 934 cm–1 (Fig. 5, curve 2), whereas the spectrum of the same solution but in the presence of hydrochloric acid shows two bands with close located frequencies at 905 and 934 cm–1.
Under resonance excitation of the solutions of monomeric and dimeric protonated forms of porphyrin, the corresponding spectra show the frequencies, which characterize vibrational modes of porphyrin and its local surrounding. Hence, a narrow band at 1014 cm–1 in the Raman spectrum (Fig. 2, curve 2) characterizes the state of water associated with monomeric porphyrin. From this viewpoint, the band at 1014 cm–1 and bands at 1000 and 1025 cm–1 observed in the spectra of dimeric forms should be assigned to proton transitions of water molecules connected with porphyrin via hydrogen bonds and simultaneously with a system of hydrogen bonds of the tetrahedral network of liquid water. According to the Raman spectra of aqueous-organic solutions (Fig. 5), a high-frequency band of the doublet located at 934 cm–1 characterizes proton transitions of neutral water molecules. Hence, the high-frequency band at 1025 cm–1 characterizes similar proton transitions of neutral water molecules connected with dimers of porphyrin. Since the low-frequency band at 905 cm–1 in the spectrum (Fig. 5, curve 3) is only observed in the presence of acid, the low-frequency band at 1000 cm–1 (Fig. 2, curve 3 and Fig. 4) should be attributed to proton transitions of hydroxonium ions, which interacts with one of molecules of dimeric porphyrin. All these bands are more narrow as compared with the similar band of a pure water because they characterize proton transitions of water molecules connected with monomeric or dimeric forms of porphyrin or an organic molecules when the bands at 905 and 934 cm–1 are observed in the spectra.
This identification of frequency lines agrees with the results obtained by other authors [13, 14] because the conjoining of proton to a water molecule increases the rotational inertia moment of this molecule as in the case of isotopic substitution of proton and, hence, decreases the frequency of rotational vibrations. At the same time, frequency of proton or deuteron transitions depends on the properties of spatial tetrahedral network or in fact on strength parameters of hydrogen bonds. In two different networks, similar deuteron transitions are characterized by different frequencies: 490 cm–1 in water-deuterium and 700 cm-1 in the network of a pure deuterium water. In aqueous-organic solutions, frequency lines of proton transitions decreases about by 65 - 100 cm–1 as compared with that of a pure water [13]. This decrease is a result of a decreased elasticity of tetrahedral network induced by addition of organic component.
It is important to note that water is connected with porphyrin even when it exists in its monomeric state. This fact is proved by the band at 1014 cm–1 and bands at 208 and 348 cm–1. The band at 208 cm–1 is likely to correspond to the similar band at 218 cm–1, which is involved in one of the doublets of the spectrum of dimeric forms of porphyrin. Whereas, the other one corresponds to the band at 354 cm–1, which is involved in the other doublet. This evidence suggests that different vibrational characteristics of water connected with porphyrin are associated with different sites of binding in a porphyrin molecule. This situation is likely to be associated with the presence of two principal conformational states of porphyrin molecules such as two NH-tautomeric forms.
Hence, the above results may be interpreted as follows. Two types of the bound water molecules are involved in the composition of solvate cover of singly and doubly protonated dimeric forms of tetraphenylporphine. The first type involves water molecules, which interact with protonated forms of nitrogen of porphyrin macrocycle as a result of which hydrogen bond between oxygen in water molecule and the protonated nitrogen is formed. Their proton transitions are characterized by the band at 1000 cm–1. The second type involves the bound water molecules, which are the molecules located at the second coordination shell of the protonated ternary nitrogen atom. At the same time, these molecules interact with a not protonated ternary nitrogen atom, so they are also involved in the first coordination cover of dimeric form of porphyrin and characterized by the band at 1025 cm–1. Hence, polarizability of hydrogen bonds formed by the two types of water molecules is responsible for unchanged characteristics of the doublet formed by the narrow band at 1000 cm–1 and somewhat broadened band at 1025 cm–1.
According to the previous results obtained for the associated forms of meso-tetra(p-aminophenyl)porphine, the band at 1607 cm–1 characterizes the donor-acceptor interaction between water and associated porphyrin when water molecules donate protons [15]. The presence of similar band at 1608 cm–1 in the Raman spectra of the protonated dimeric forms of tetraphenylporphine suggests that the protonated dimers interact with water in a similar way so a bound water molecule donates proton to porphyrin molecule. As a result, the
p-electron structure of dimeric monocation of porphyrin is stabilized with the formation of donor-acceptor complex between the molecules of porphyrin and water.Electron structure of monocation of the monomeric porphyrin is unstable under usual conditions whereas, in the case of porphyrin dimer, there is a possibility for stabilization of the
p-electron structure of porphyrin macrocycle. According to the results, stabilization of the p-electron structure of macrocycle in dimeric monocation can occur via its interaction with one water molecule, which lends electron to the protonated nitrogen atom and proton to the neighboring porphyrin molecule. Another water molecule forms hydrogen bonds with ternary nitrogen atoms of these porphyrin molecules because stabilization of p-electron structure of dimeric monocation requires addition of second proton or an electron. Hence, neighboring porphyrin molecules in dimer are coordinated via two water molecules with the formation of hydrogen bonds between them. It appears that the water molecule, which forms hydrogen bonds, may be considered as a neutral one, whereas another water molecule is involved in the donor-acceptor interaction with porphyrin. As a result, water and porphyrin in this complex are involved in the donor-acceptor interaction and at the same time the interaction does not destroy the tetrahedral network of hydrogen bonds. This situation restricts the freedom of vibrations of the bound water molecules and provides the existence of fine structure in the region of translational vibrations of water in the Raman spectrum of dimeric porphyrin under excitation with blue light. In the case of the doubly protonated dimer, perhaps two water molecules are involved in the donor-acceptor interaction with porphyrin. However, both protons are likely to be connected with one of the neighboring porphyrin molecules in dimer because of the similar stabilization of the p-electron structure of porphyrin macrocycle.The results of Raman spectroscopy confirm the above speculations concerning the interaction of dimeric forms of porphyrin with water. The blue light excitation of protonated dimers should lead to the activation of selective translational vibrations of water connected with dimeric forms since under this condition the energy of exciton interactions equaled to 1650 cm–1 is very close to the energy of deformational vibrations of water [16]. The bands with maxima at 218, 245, 328, and 353 cm–1 just evidence the occurrence of selective translational vibrations of water connected with protonated dimers of porphyrin. Note that these bands form two doublets with the same interval between neighboring maxima, which equals to 25 ± 2 cm–1. Hence, doublet character of the bands found for proton transitions are revealed in the region of translational vibrations of water too.
Analysis of the results of absorption and Raman spectroscopy allows one to advance a structural model for the singly protonated dimer of porphyrin, which takes into account the above specific features. Figure 6 shows the structure of this water-porphyrin complex. As follows from Fig. 6, two porphyrin molecules form a dimer when two water molecules are involved in this dimer with the formation of hydrogen bonds between them. The existence of charge on one porphyrin molecule leads to a strong polarization of water molecule bound with the porphyrin. At the same time, as a result of NH-tautomeric transitions, migration of proton in this structure of porphyrin dimer may provide a limited set of its conformational states, which control a set of translational modes of water molecules involved in this water-porphyrin complex.
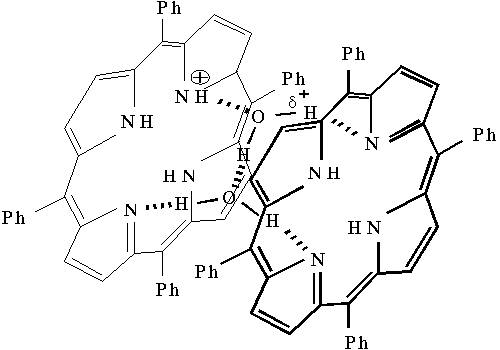
Fig. 6. The structure for proposed singly protonated meso-tetraphenylporphine dimer-water complex in aqueous-organic solutions.
Hence, interaction of porphyrin with water provides a direct involvement of water molecules in the structure of dimeric complex and formation of a stable
p-electron configuration of porphyrin macrocycle.Conclusion
The presented results show that the bands at 1000 and 1025 ± 2 cm–1 in the resonance Raman spectra of singly and doubly protonated dimeric forms of tetraphenylporphine characterize the proton transitions in water molecules and hydroxonium ions connected with the dimeric forms. These spectra show similar doublet character of the bands with an interval of 25 ± 2 cm–1 in the region of translational vibrations of water. Selective activation of the vibrations of water connected with porphyrin dimers suggests an existence of a limited set of configurations of the water-porphyrin dimeric complex. In contrast, in this region of the Raman spectra of the corresponding aqueous-organic solutions, one may see a broad structureless band, which characterizes free vibrations of water in the structure of spatial tetrahedral network of its hydrogen bonds. Analysis of vibrational characteristics of the dimeric forms of singly protonated tetraphenylporphine allows to advance the structure of the dimeric complex with water.
According to the experimental evidence obtained, stabilization of the
p-electron structure of the porphyrin macrocycle of the dimeric monocation is possible via accepting by the neighboring porphyrin of proton of the water molecule connected with the monocation and subsequent porphyrin coordination with the formation of hydrogen bonds between the porphyrin molecules. As a result, water and porphyrin are involved in the donor-acceptor interaction, and this peculiarity is responsible for the appearance of the fine structure in the region of translational vibrations of water.Acknowledgement
This work was supported by the Russian Foundation for Basic Research, project No. 97-04-48155.
References
1 F.F. Litvin, and V.A. Sineshchekov. In Bioenergetics of Photosynthesis, ed. by Govindjee, Academic Press, New York 1975, pp. 619 - 661.
2 J.J. Katz, L.L. Shipman, T.M. Cotton, and T.R. Janson. In The Porphyrins, ed. by D. Dolphin, Academic Press, New York 1979, V. 5, pp. 401 - 458.
3 V.A. Shuvalov, Primary steps of light energy conversion in photosynthesis. Nauka, Moscow, 1990 (in Russian).
4 M.R. Wasielewski, Chem. Rev. 92 (1992) 435 - 461.
5 A.B. Rubin, A.A. Kononenko, V.Z. Paschenko, B.A. Guliaev, C.K. Chamorovsky. Proceedings of Science and Techniques, Biophysics, WINITI, Moscow 1987, V. 20, pp. 246 (in Russian).
6 Photosynthesis. Chemical models and mechanisms, ed. by V.M. Cherkasov, Nauk. Dumka, Kiev 1989, pp. 227 (in Russian).
7 A.V.Udal'tsov, L.A. Kazarin. J. Photochem. and Photobiol. A: Chem. 96 (1996) 99 - 107.
8 A.V.Udal'tsov. Biochemistry (Moscow), 62 (1997) 1026 - 1033.
9 A.D. Adler, F.R. Longo, J.D. Finarelli, J. Goldmacher, J. Assour, and L. Korsakoff. J. Org. Chem. 32 (1967) 476.
10 A. Gordon, and R. Ford. Sputnik Khimika, Mir, Moscow 1976, p.437 (in Russian).
11 Z. I. Gadgiev, A. A. Churin, V. Z. Paschenko, and L. B. Rubin. Biol. Nauki, 8 (1980) 98 - 104 (in Russian).
12 D. Eisenbeerg and W. Kauzmann. The Structure and Properties of Water, Oxford University Press, Oxford 1969, pp. 282.
13 J.R. Scherer, R.G. Snyder. J. Chem. Phys. 67 (1977) 4794 - 4811.
14 G.N. Zatsepina, Physical Properties and Structure of Water. Moscow State University Press, Moscow 1987 (in Russian).
15 A.V. Udal'tsov and L.A. Kazarin, Biochemistry (Moscow) 61 (1996) 367 - 373.
16 A.V. Udal'tsov, A.A. Churin, Internet Photochem. Photobiol. 1998, http:// www.photobiology.com./v1/udaltsov/udaltsov.htm